Ecological Adaptations of Gut Microbiota Members and Their Consequences for Use as a New Generation of Probiotics
- PMID: 34067354
- PMCID: PMC8196900
- DOI: 10.3390/ijms22115471
Ecological Adaptations of Gut Microbiota Members and Their Consequences for Use as a New Generation of Probiotics
Abstract
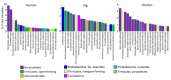
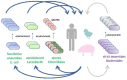
In this review, we link ecological adaptations of different gut microbiota members with their potential for use as a new generation of probiotics. Gut microbiota members differ in their adaptations to survival in aerobic environments. Interestingly, there is an inverse relationship between aerobic survival and abundance or potential for prolonged colonization of the intestinal tract. Facultative anaerobes, aerotolerant Lactobacilli and endospore-forming Firmicutes exhibit high fluctuation, and if such bacteria are to be used as probiotics, they must be continuously administered to mimic their permanent supply from the environment. On the other hand, species not expressing any form of aerobic resistance, such as those from phylum Bacteroidetes, commonly represent host-adapted microbiota members characterized by vertical transmission from mothers to offspring, capable of long-term colonization following a single dose administration. To achieve maximal probiotic efficacy, the mode of their administration should thus reflect their natural ecology.
Keywords: chicken; gut; human; microbiota; pig; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wu Y., Li S., Tao Y., Li D., Han Y., Show P.L., Wen G., Zhou J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021;348:129083. doi: 10.1016/j.foodchem.2021.129083. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

