Remdesivir for Early COVID-19 Treatment of High-Risk Individuals Prior to or at Early Disease Onset-Lessons Learned
- PMID: 34067381
- PMCID: PMC8224666
- DOI: 10.3390/v13060963
Remdesivir for Early COVID-19 Treatment of High-Risk Individuals Prior to or at Early Disease Onset-Lessons Learned
Abstract
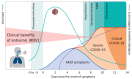
After more than one year of the COVID-19 pandemic, antiviral treatment options against SARS-CoV-2 are still severely limited. High hopes that had initially been placed on antiviral drugs like remdesivir have so far not been fulfilled. While individual case reports provide striking evidence for the clinical efficacy of remdesivir in the right clinical settings, major trials failed to demonstrate this. Here, we highlight and discuss the key findings of these studies and underlying reasons for their failure. We elaborate on how such shortcomings should be prevented in future clinical trials and pandemics. We suggest in conclusion that any novel antiviral agent that enters human trials should first be tested in a post-exposure setting to provide rapid and solid evidence for its clinical efficacy before initiating further time-consuming and costly clinical trials for more advanced disease. In the COVID-19 pandemic this might have established remdesivir early on as an efficient antiviral agent at a more suitable disease stage which would have saved many lives, in particular in large outbreaks within residential care homes.
Keywords: COVID-19; SARS-CoV-2; antiviral treatment; remdesivir.
Conflict of interest statement
C.D.S. reports grants, personal fees and non-financial support from Gilead Sciences, grants and personal fees from Janssen-Cilag, personal fees from Formycon, other from Apeiron, other from Eli Lilly, during the conduct of the study; personal fees from AbbVie, personal fees from MSD, grants and personal fees from GSK/ViiV Healthcare, outside the submitted work. L.D. and A.S. report no conflict of interest.
Figures
Similar articles
-
Remdesivir: A beacon of hope from Ebola virus disease to COVID-19.Rev Med Virol. 2020 Nov;30(6):1-13. doi: 10.1002/rmv.2133. Epub 2020 Jul 30. Rev Med Virol. 2020. PMID: 33210457 Review.
-
Remdesivir in Coronavirus Disease 2019 (COVID-19) treatment: a review of evidence.Infection. 2021 Jun;49(3):401-410. doi: 10.1007/s15010-020-01557-7. Epub 2021 Jan 2. Infection. 2021. PMID: 33389708 Free PMC article. Review.
-
Remdesivir for the treatment of COVID 19: review of the pharmacological properties, safety and clinical effectiveness.Expert Opin Drug Saf. 2021 Nov;20(11):1299-1307. doi: 10.1080/14740338.2021.1962284. Epub 2021 Aug 16. Expert Opin Drug Saf. 2021. PMID: 34338121 Review.
-
Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection.Emerg Microbes Infect. 2021 Dec;10(1):291-304. doi: 10.1080/22221751.2021.1885998. Emerg Microbes Infect. 2021. PMID: 33538646 Free PMC article.
-
Persistent replication of SARS-CoV-2 in a severely immunocompromised patient treated with several courses of remdesivir.Int J Infect Dis. 2021 Mar;104:379-381. doi: 10.1016/j.ijid.2020.12.050. Epub 2020 Dec 21. Int J Infect Dis. 2021. PMID: 33359065 Free PMC article.
Cited by
-
SARS-CoV-2 viral load dynamics in immunocompromised critically ill patients on remdesivir treatment.Multidiscip Respir Med. 2022 May 10;17:825. doi: 10.4081/mrm.2022.825. eCollection 2022 Jan 12. Multidiscip Respir Med. 2022. PMID: 35646346 Free PMC article.
-
Sensing of SARS-CoV-2 by pDCs and their subsequent production of IFN-I contribute to macrophage-induced cytokine storm during COVID-19.Sci Immunol. 2022 Sep 9;7(75):eadd4906. doi: 10.1126/sciimmunol.add4906. Epub 2022 Sep 9. Sci Immunol. 2022. PMID: 36083891 Free PMC article.
-
Identification of novel SARS-CoV-2 RNA dependent RNA polymerase (RdRp) inhibitors: From in silico screening to experimentally validated inhibitory activity.Comput Struct Biotechnol J. 2022;20:882-890. doi: 10.1016/j.csbj.2022.02.001. Epub 2022 Feb 4. Comput Struct Biotechnol J. 2022. PMID: 35136534 Free PMC article.
-
Association of Treatment with Remdesivir and 30-day Hospital Readmissions in Patients Hospitalized with COVID-19.Am J Med Sci. 2022 May;363(5):403-410. doi: 10.1016/j.amjms.2022.01.021. Epub 2022 Feb 10. Am J Med Sci. 2022. PMID: 35151637 Free PMC article.
-
Optimizing antiviral therapy for COVID-19 with learned pathogenic model.Sci Rep. 2022 Apr 27;12(1):6873. doi: 10.1038/s41598-022-10929-y. Sci Rep. 2022. PMID: 35477965 Free PMC article.
References
-
- Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. - DOI - PMC - PubMed
-
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9:e00221-18. doi: 10.1128/mBio.00221-18. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

