Effects of Apigenin and Astragalus Polysaccharide on the Cryopreservation of Bull Semen
- PMID: 34067384
- PMCID: PMC8224660
- DOI: 10.3390/ani11061506
Effects of Apigenin and Astragalus Polysaccharide on the Cryopreservation of Bull Semen
Abstract
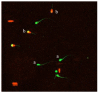
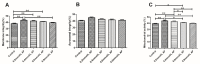
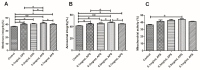
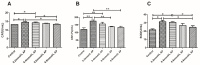
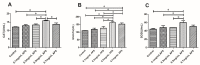
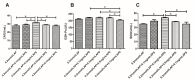
The purpose of this study was to determine the effects of apigenin and astragalus polysaccharides on the cryopreservation of bovine semen. Apigenin, astragalus polysaccharides, or their combination were added to a frozen diluent of bovine semen. Afterwards, Computer Assisted Semen Analysis (CASA), membrane functionality, acrosome integrity, mitochondrial integrity, CAT, SOD, GSH-Px, MDA, and ROS detection were conducted. The results showed that adding 0.2 mmol/L AP or 0.5 mg/mL APS could improve the quality of frozen sperm. Compared to 0.2 mmol/L AP alone, the combination of 0.2 mmol/L AP and 0.3 mg/mL APS significantly increased the total motility (TM), average path distance (DAP), straight line distance (DSL), average path velocity (VAP), curvilinear velocity (VCL), wobble (WOB), and sperm CAT and SOD levels (p < 0.05), while reducing the ROS and MDA levels (p < 0.05). These results indicated that the addition of 0.2 mmol/L AP or 0.5 mg/mL APS alone has a protective effect on the freezing of bovine semen. Compared to the addition of 0.2 mmol/L AP, a combination of 0.2 mmol/L AP and 0.3 mg/mL APS could further improve the quality of frozen semen.
Keywords: apigenin; astragalus polysaccharide; bull semen; cryopreservation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Oligomeric Proanthocyanidins and Bamboo Leaf Flavonoids Improve the Quality of Bull Semen Cryopreservation.Molecules. 2022 Feb 8;27(3):1144. doi: 10.3390/molecules27031144. Molecules. 2022. PMID: 35164407 Free PMC article.
-
Epigallocatechin 3-gallate improves the quality of bull semen cryopreservation.Andrologia. 2022 Feb;54(1):e14310. doi: 10.1111/and.14310. Epub 2021 Nov 8. Andrologia. 2022. PMID: 34750852
-
Effect of Glutathione on Sperm Quality in Guanzhong Dairy Goat Sperm During Cryopreservation.Front Vet Sci. 2021 Nov 18;8:771440. doi: 10.3389/fvets.2021.771440. eCollection 2021. Front Vet Sci. 2021. PMID: 34869742 Free PMC article.
-
Effects of Different Diluents and Freezing Methods on Cryopreservation of Hu Ram Semen.Vet Sci. 2024 Jun 3;11(6):251. doi: 10.3390/vetsci11060251. Vet Sci. 2024. PMID: 38921998 Free PMC article.
-
Effect of Cryopreservation on Casa Characteristics, Mitochondrial Transmembrane Potential, Plasma and Acrosome Integrities, Morphology and in vivo Fertility of Buffalo Bull Spermatozoa.Cryo Letters. 2019 May/Jun;40(3):173-180. Cryo Letters. 2019. PMID: 31095666
Cited by
-
Apigenin supplementation substantially improves rooster sperm freezability and post-thaw function.Sci Rep. 2024 Feb 24;14(1):4527. doi: 10.1038/s41598-024-55057-x. Sci Rep. 2024. PMID: 38402367 Free PMC article.
-
Astragalus polysaccharides protect against Di-n-butyl phthalate-induced testicular damage by modulating oxidative stress, apoptosis, and the PI3K/Akt/mTOR pathway in rats.Front Vet Sci. 2025 Jul 28;12:1616186. doi: 10.3389/fvets.2025.1616186. eCollection 2025. Front Vet Sci. 2025. PMID: 40792058 Free PMC article.
-
Dietary supplementation of Astragalus polysaccharide or its nanoparticles enhances testicular hemodynamics, echotexture, scrotal circumference, concentration of testosterone, estradiol, nitric oxide, and total antioxidant capacity, and semen quality in mature Ossimi rams.BMC Vet Res. 2025 Feb 6;21(1):55. doi: 10.1186/s12917-025-04477-6. BMC Vet Res. 2025. PMID: 39915778 Free PMC article.
-
Supplementation with MitoTEMPO before cryopreservation improves sperm quality and fertility potential of Piedmontese beef bull semen.Front Vet Sci. 2024 May 15;11:1376057. doi: 10.3389/fvets.2024.1376057. eCollection 2024. Front Vet Sci. 2024. PMID: 38812559 Free PMC article.
-
Examining the combined benefits of photobiomodulation and apigenin for the treatment of asthenozoospermia: An innovative therapeutic strategy.Photochem Photobiol Sci. 2024 Oct;23(10):1945-1955. doi: 10.1007/s43630-024-00643-1. Epub 2024 Oct 5. Photochem Photobiol Sci. 2024. PMID: 39367935
References
-
- de Lamirande E., Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992;13:368–378. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

