Hemisynthesis and Biological Evaluation of Cinnamylated, Benzylated, and Prenylated Dihydrochalcones from a Common Bio-Sourced Precursor
- PMID: 34067407
- PMCID: PMC8224620
- DOI: 10.3390/antibiotics10060620
Hemisynthesis and Biological Evaluation of Cinnamylated, Benzylated, and Prenylated Dihydrochalcones from a Common Bio-Sourced Precursor
Abstract
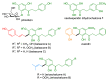
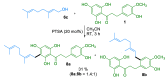
Several families of naturally occurring C-alkylated dihydrochalcones display a broad range of biological activities, including antimicrobial and cytotoxic properties, depending on their alkylation sidechain. The catalytic Friedel-Crafts alkylation of the readily available aglycon moiety of neohesperidin dihydrochalcone was performed using cinnamyl, benzyl, and isoprenyl alcohols. This procedure provided a straightforward access to a series of derivatives that were structurally related to natural balsacones, uvaretin, and erioschalcones, respectively. The antibacterial and cytotoxic potential of these novel analogs was evaluated in vitro and highlighted some relations between the structure and the pharmacological properties of alkylated dihydrochalcones.
Keywords: Brønsted acid catalysis; Friedel–Crafts alkylation; Staphylococcus aureus; antibacterial; dihydrochalcone; hemisynthesis; natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Snijman P.W., Joubert E., Ferreira D., Li X.-C., Ding Y., Green I.R., Gelderblom W.C.A. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009;57:6678–6684. doi: 10.1021/jf901417k. - DOI - PubMed
-
- Lavoie S., Legault J., Simard F., Chiasson É., Pichette A. New antibacterial dihydrochalcone derivatives from buds of Populus balsamifera. Tetrahedron Lett. 2013;54:1631–1633. doi: 10.1016/j.tetlet.2012.12.012. - DOI
LinkOut - more resources
Full Text Sources