Translational Studies on the Potential of a VEGF Nanoparticle-Loaded Hyaluronic Acid Hydrogel
- PMID: 34067451
- PMCID: PMC8224549
- DOI: 10.3390/pharmaceutics13060779
Translational Studies on the Potential of a VEGF Nanoparticle-Loaded Hyaluronic Acid Hydrogel
Abstract
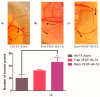
Heart failure has a five-year mortality rate approaching 50%. Inducing angiogenesis following a myocardial infarction is hypothesized to reduce cardiomyocyte death and tissue damage, thereby preventing heart failure. Herein, a novel nano-in-gel delivery system for vascular endothelial growth factor (VEGF), composed of star-shaped polyglutamic acid-VEGF nanoparticles in a tyramine-modified hyaluronic acid hydrogel (nano-VEGF-HA-TA), is investigated. The ability of the nano-VEGF-HA-TA system to induce angiogenesis is assessed in vivo using a chick chorioallantoic membrane model (CAM). The formulation is then integrated with a custom-made, clinically relevant catheter suitable for minimally invasive endocardial delivery and the effect of injection on hydrogel properties is examined. Nano-VEGF-HA-TA is biocompatible on a CAM assay and significantly improves blood vessel branching (p < 0.05) and number (p < 0.05) compared to a HA-TA hydrogel without VEGF. Nano-VEGF-HA-TA is successfully injected through a 1.2 m catheter, without blocking or breaking the catheter and releases VEGF for 42 days following injection in vitro. The released VEGF retains its bioactivity, significantly improving total tubule length on a Matrigel® assay and human umbilical vein endothelial cell migration on a Transwell® migration assay. This VEGF-nano in a HA-TA hydrogel delivery system is successfully integrated with an appropriate device for clinical use, demonstrates promising angiogenic properties in vivo and is suitable for further clinical translation.
Keywords: angiogenic growth factor; catheter delivery; chick chorioallantoic membrane model; hyaluronic acid hydrogel; nanoparticle-loaded hydrogel; protein delivery; sustained release; vascular endothelial growth factor nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest. Authors L.K., M.P. and V.V. are employees of Contipro. The funders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Figures








References
-
- National Institute for Health and Clinical Excellence . National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. Royal College of Physicians; London, UK: 2010. Chronic Heart Failure. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

