Plasma Electrolytic Oxidation (PEO) Process-Processing, Properties, and Applications
- PMID: 34067483
- PMCID: PMC8224744
- DOI: 10.3390/nano11061375
Plasma Electrolytic Oxidation (PEO) Process-Processing, Properties, and Applications
Abstract
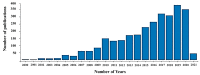
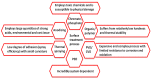
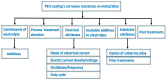
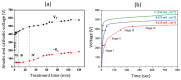
Plasma electrolytic oxidation (PEO) is a novel surface treatment process to produce thick, dense metal oxide coatings, especially on light metals, primarily to improve their wear and corrosion resistance. The coating manufactured from the PEO process is relatively superior to normal anodic oxidation. It is widely employed in the fields of mechanical, petrochemical, and biomedical industries, to name a few. Several investigations have been carried out to study the coating performance developed through the PEO process in the past. This review attempts to summarize and explain some of the fundamental aspects of the PEO process, mechanism of coating formation, the processing conditions that impact the process, the main characteristics of the process, the microstructures evolved in the coating, the mechanical and tribological properties of the coating, and the influence of environmental conditions on the coating process. Recently, the PEO process has also been employed to produce nanocomposite coatings by incorporating nanoparticles in the electrolyte. This review also narrates some of the recent developments in the field of nanocomposite coatings with examples and their applications. Additionally, some of the applications of the PEO coatings have been demonstrated. Moreover, the significance of the PEO process, its current trends, and its scope of future work are highlighted.
Keywords: additives; corrosion; nanocomposite coating; plasma electrolytic oxidation; tribology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures














Similar articles
-
Protecting Light Metal Alloys Using a Sustainable Plasma Electrolytic Oxidation Process.ACS Omega. 2022 Mar 2;7(10):8570-8580. doi: 10.1021/acsomega.1c06442. eCollection 2022 Mar 15. ACS Omega. 2022. PMID: 35309481 Free PMC article.
-
Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate.Materials (Basel). 2021 Jul 19;14(14):4037. doi: 10.3390/ma14144037. Materials (Basel). 2021. PMID: 34300956 Free PMC article.
-
Advances in amelioration of plasma electrolytic oxidation coatings on biodegradable magnesium and alloys.Heliyon. 2024 Jan 11;10(4):e24348. doi: 10.1016/j.heliyon.2024.e24348. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38434039 Free PMC article. Review.
-
Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding.Materials (Basel). 2022 Nov 22;15(23):8300. doi: 10.3390/ma15238300. Materials (Basel). 2022. PMID: 36499797 Free PMC article.
-
Surface modification of valve metals using plasma electrolytic oxidation for antibacterial applications: A review.J Biomed Mater Res A. 2018 Feb;106(2):590-605. doi: 10.1002/jbm.a.36259. Epub 2017 Oct 30. J Biomed Mater Res A. 2018. PMID: 28975693 Review.
Cited by
-
Influence of silicon morphology on direct current plasma electrolytic oxidation process in AlSi10Mg alloy produced with laser powder bed fusion.Sci Rep. 2022 Aug 22;12(1):14329. doi: 10.1038/s41598-022-18176-x. Sci Rep. 2022. PMID: 35995994 Free PMC article.
-
Advancements in Surface Modification of NiTi Alloys for Orthopedic Implants: Focus on Low-Temperature Glow Discharge Plasma Oxidation Techniques.Int J Mol Sci. 2025 Jan 28;26(3):1132. doi: 10.3390/ijms26031132. Int J Mol Sci. 2025. PMID: 39940898 Free PMC article. Review.
-
Theoretical and experimental investigation of a CuO and graphene embedded polyethylene oxide counter electrode for efficient DSSCs.Sci Rep. 2025 Jul 11;15(1):25049. doi: 10.1038/s41598-025-98930-z. Sci Rep. 2025. PMID: 40646160 Free PMC article.
-
The effect of silica NPs incorporation on protective properties of oxide layers formed by PEO on Mg97Y2Zn1 alloy with LPSO-phase.Heliyon. 2023 Nov 15;9(11):e22435. doi: 10.1016/j.heliyon.2023.e22435. eCollection 2023 Nov. Heliyon. 2023. PMID: 38058629 Free PMC article.
-
Magnesium-Titanium Alloys: A Promising Solution for Biodegradable Biomedical Implants.Materials (Basel). 2024 Oct 23;17(21):5157. doi: 10.3390/ma17215157. Materials (Basel). 2024. PMID: 39517433 Free PMC article. Review.
References
-
- Lu X., Mohedano M., Blawert C., Matykina E., Arrabal R., Kainer K.U., Zheludkevich M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016;307:1165–1182. doi: 10.1016/j.surfcoat.2016.08.055. - DOI
-
- Kaseem M., Fatimah S., Nashrah N., Ko Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2020;117:100735. doi: 10.1016/j.pmatsci.2020.100735. - DOI
-
- Rapheal G., Kumar S., Scharnagl N., Blawert C. Effect of current density on the microstructure and corrosion properties of plasma electrolytic oxidation (PEO) coatings on AM50 Mg alloy produced in an electrolyte containing clay additives. Surf. Coat. Technol. 2016;289:150–164. doi: 10.1016/j.surfcoat.2016.01.033. - DOI
-
- Sluginov N. On luminous phenomen, observed in liquids during electrolysis. Russ. Phys. Chem. Soc. 1880;12:193–203.
-
- Simchen F., Sieber M., Kopp A., Lampke T. Introduction to plasma electrolytic oxidation—An overview of the process and applications. Coatings. 2020;10:628. doi: 10.3390/coatings10070628. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

