Bacterial Resistance to Antimicrobial Agents
- PMID: 34067579
- PMCID: PMC8157006
- DOI: 10.3390/antibiotics10050593
Bacterial Resistance to Antimicrobial Agents
Abstract
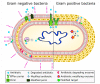
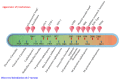
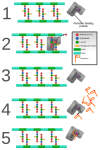
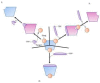
Bacterial pathogens as causative agents of infection constitute an alarming concern in the public health sector. In particular, bacteria with resistance to multiple antimicrobial agents can confound chemotherapeutic efficacy towards infectious diseases. Multidrug-resistant bacteria harbor various molecular and cellular mechanisms for antimicrobial resistance. These antimicrobial resistance mechanisms include active antimicrobial efflux, reduced drug entry into cells of pathogens, enzymatic metabolism of antimicrobial agents to inactive products, biofilm formation, altered drug targets, and protection of antimicrobial targets. These microbial systems represent suitable focuses for investigation to establish the means for their circumvention and to reestablish therapeutic effectiveness. This review briefly summarizes the various antimicrobial resistance mechanisms that are harbored within infectious bacteria.
Keywords: antimicrobial resistance; bacteria; infection; multidrug resistance; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kumar S., Varela M.F. Molecular mechanisms of bacterial resistance to antimicrobial agents. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education. In: Méndez-Vilas A., editor. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education. Formatex Research Center; Badajoz, Spain: 2013. pp. 522–534.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

