Predicting the Adsorption of Amoxicillin and Ibuprofen on Chitosan and Graphene Oxide Materials: A Density Functional Theory Study
- PMID: 34067695
- PMCID: PMC8156938
- DOI: 10.3390/polym13101620
Predicting the Adsorption of Amoxicillin and Ibuprofen on Chitosan and Graphene Oxide Materials: A Density Functional Theory Study
Abstract
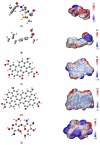
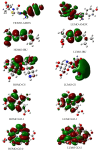
The occurrence, persistence, and accumulation of antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs) represent a new environmental problem due to their harmful effects on human and aquatic life. A suitable absorbent for a particular type of pollutant does not necessarily absorb other types of compounds, so knowing the compatibility between a particular pollutant and a potential absorbent before experimentation seems to be fundamental. In this work, the molecular interactions between some pharmaceuticals (amoxicillin, ibuprofen, and tetracycline derivatives) with two potential absorbers, chitosan and graphene oxide models (pyrene, GO-1, and coronene, GO-2), were studied using the ωB97X-D/6-311G(2d,p) level of theory. The energetic interaction order found was amoxicillin/chitosan > amoxicillin/GO-1 > amoxicillin/GO-2 > ibuprofen/chitosan > ibuprofen/GO-2 > ibuprofen/GO-1, the negative sign for the interaction energy in all complex formations confirms good compatibility, while the size of Eint between 24-34 kcal/mol indicates physisorption processes. Moreover, the free energies of complex formation were negative, confirming the spontaneity of the processes. The larger interaction of amoxicillin Gos, compared to ibuprofen Gos, is consistent with previously reported experimental results, demonstrating the exceptional predictability of these methods. The second-order perturbation theory analysis shows that the amoxicillin complexes are mainly driven by hydrogen bonds, while van der Waals interactions with chitosan and hydrophobic interactions with graphene oxides are modelled for the ibuprofen complexes. Energy decomposition analysis (EDA) shows that electrostatic energy is a major contributor to the stabilization energy in all cases. The results obtained in this work promote the use of graphene oxides and chitosan as potential adsorbents for the removal of these emerging pollutants from water.
Keywords: absorption; density functional theory; emergent pollutants; natural bond orbital; pharmaceuticals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Investigating the structure of the product of graphene oxide reaction with folic acid and chitosan: density functional theory calculations.J Biomol Struct Dyn. 2022;40(24):14146-14159. doi: 10.1080/07391102.2021.2001372. Epub 2021 Nov 18. J Biomol Struct Dyn. 2022. PMID: 34791994
-
Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite.Carbohydr Polym. 2019 Sep 15;220:141-148. doi: 10.1016/j.carbpol.2019.05.060. Epub 2019 May 24. Carbohydr Polym. 2019. PMID: 31196533
-
Graphene-Based Materials Immobilized within Chitosan: Applications as Adsorbents for the Removal of Aquatic Pollutants.Materials (Basel). 2021 Jun 30;14(13):3655. doi: 10.3390/ma14133655. Materials (Basel). 2021. PMID: 34209007 Free PMC article. Review.
-
Removal of caffeine, nicotine and amoxicillin from (waste)waters by various adsorbents. A review.J Environ Manage. 2020 May 1;261:110236. doi: 10.1016/j.jenvman.2020.110236. Epub 2020 Mar 2. J Environ Manage. 2020. PMID: 32148306 Review.
-
Theoretical study of the adsorption of analgesic environmental pollutants on pristine and nitrogen-doped graphene nanosheets.Phys Chem Chem Phys. 2021 Jan 21;23(2):1221-1233. doi: 10.1039/d0cp05543c. Phys Chem Chem Phys. 2021. PMID: 33355576
Cited by
-
Molecular Modeling of Vasodilatory Activity: Unveiling Novel Candidates Through Density Functional Theory, QSAR, and Molecular Dynamics.Int J Mol Sci. 2024 Nov 25;25(23):12649. doi: 10.3390/ijms252312649. Int J Mol Sci. 2024. PMID: 39684360 Free PMC article.
-
Adsorption of Organic Pollutants from Wastewater Using Chitosan-Based Adsorbents.Polymers (Basel). 2025 Feb 14;17(4):502. doi: 10.3390/polym17040502. Polymers (Basel). 2025. PMID: 40006164 Free PMC article. Review.
-
[Surface-enhanced Raman detection of deoxynivalenol allenol in agricultural products].Se Pu. 2022 Nov;40(11):1039-1046. doi: 10.3724/SP.J.1123.2022.06021. Se Pu. 2022. PMID: 36351813 Free PMC article. Chinese.
-
Influence of β-Cyclodextrin Methylation on Host-Guest Complex Stability: A Theoretical Study of Intra- and Intermolecular Interactions as Well as Host Dimer Formation.Molecules. 2023 Mar 14;28(6):2625. doi: 10.3390/molecules28062625. Molecules. 2023. PMID: 36985598 Free PMC article.
-
Green decoration of graphene oxide Nano sheets with gelatin and gum Arabic for targeted delivery of doxorubicin.Biotechnol Rep (Amst). 2022 Mar 18;34:e00722. doi: 10.1016/j.btre.2022.e00722. eCollection 2022 Jun. Biotechnol Rep (Amst). 2022. PMID: 35686004 Free PMC article.
References
-
- Barker K. Biosecure citizenship: Politicising symbiotic associations and the construction of biological threat. Trans. Inst. Br. Geogr. 2010;35:350–363. doi: 10.1111/j.1475-5661.2010.00386.x. - DOI
-
- Biermann C., Anderson R.M. Conservation, biopolitics, and the governance of life and death. Geogr. Compass. 2017;11:e12329. doi: 10.1111/gec3.12329. - DOI
-
- Dempsey J. Enterprising Nature: Economics, Markets and Finance in Global Biodiversity Politics. Wiley Blackwell; Hoboken, NJ, USA: 2016.
-
- Tortajada C. Contributions of recycled wastewater to clean water and sanitation Sustainable Development Goals. NPJ Clean Water. 2020;3 doi: 10.1038/s41545-020-0069-3. - DOI
-
- Bunke D., Moritz S., Brack W., Herráez D.L., Posthuma L., Nuss M. Developments in society and implications for emerging pollutants in the aquatic environment. Environ. Sci. Eur. 2019;31:1–17. doi: 10.1186/s12302-019-0213-1. - DOI
LinkOut - more resources
Full Text Sources

