Partial Rescue of F508del-CFTR Stability and Trafficking Defects by Double Corrector Treatment
- PMID: 34067708
- PMCID: PMC8156943
- DOI: 10.3390/ijms22105262
Partial Rescue of F508del-CFTR Stability and Trafficking Defects by Double Corrector Treatment
Abstract
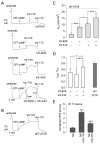
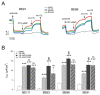
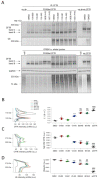
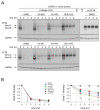
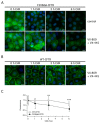
Deletion of phenylalanine at position 508 (F508del) in the CFTR chloride channel is the most frequent mutation in cystic fibrosis (CF) patients. F508del impairs the stability and folding of the CFTR protein, thus resulting in mistrafficking and premature degradation. F508del-CFTR defects can be overcome with small molecules termed correctors. We investigated the efficacy and properties of VX-445, a newly developed corrector, which is one of the three active principles present in a drug (Trikafta®/Kaftrio®) recently approved for the treatment of CF patients with F508del mutation. We found that VX-445, particularly in combination with type I (VX-809, VX-661) and type II (corr-4a) correctors, elicits a large rescue of F508del-CFTR function. In particular, in primary bronchial epithelial cells of CF patients, the maximal rescue obtained with corrector combinations including VX-445 was close to 60-70% of CFTR function in non-CF cells. Despite this high efficacy, analysis of ubiquitylation, resistance to thermoaggregation, protein half-life, and subcellular localization revealed that corrector combinations did not fully normalize F508del-CFTR behavior. Our study indicates that it is still possible to further improve mutant CFTR rescue with the development of corrector combinations having maximal effects on mutant CFTR structural and functional properties.
Keywords: VX-445; allosteric folding correction; chloride secretion; conformational stability; elexacaftor; primary bronchial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

