Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications
- PMID: 34067723
- PMCID: PMC8157037
- DOI: 10.3390/nano11051316
Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications
Abstract
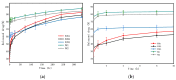
The phenomenon of drug burst release is the main problem in the field of drug delivery systems, as it means that a good therapeutic effect cannot be acheived. Nanofibers developed by electrospinning technology have large specific surface areas, high porosity, and easily controlled morphology. They are being considered as potential carriers for sustained drug release. In this paper, we obtained polycaprolactone (PCL)/polylactic acid (PLA) core-shell porous drug-carrying nanofibers by using coaxial electrospinning technology and the nonsolvent-induced phase separation method. Roxithromycin (ROX), a kind of antibacterial agent, was encapsulated in the core layer. The morphology, composition, and thermal properties of the resultant nanofibers were characterized by scanning electron microscopy (SEM), attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy, differential scanning calorimetry (DSC) and thermogravimetry analysis (TGA). Besides this, the in vitro drug release profile was investigated; it showed that the release rate of the prepared coaxial porous nanofibers with two different pore sizes was 30.10 ± 3.51% and 35.04 ± 1.98% in the first 30 min, and became 92.66 ± 3.13% and 88.94 ± 1.58% after 14 days. Compared with the coaxial nonporous nanofibers and nanofibers prepared by uniaxial electrospinning with or without pores, the prepared coaxial porous nanofibers revealed that the burst release was mitigated and the dissolution rate of the hydrophobic drugs was increased. The further antimicrobial activity demonstrated that the inhibition zone diameter of the coaxial nanofibers with two different pore sizes was 1.70 ± 0.10 cm and 1.73 ± 0.23 cm, exhibiting a good antibacterial effect against Staphylococcus aureus. Therefore, the prepared nanofibers with the coaxial porous structures could serve as promising drug delivery systems.
Keywords: PCL/PLA; antibacterial activity; coaxial electrospinning; drug release; phase separation; porous nanofibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Electrospun nanofibers of curcumin/HP-beta-CD/pullulan complex with enhanced solubility and controlled release in food and drug delivery applications.Int J Biol Macromol. 2025 Apr;300:140064. doi: 10.1016/j.ijbiomac.2025.140064. Epub 2025 Jan 19. Int J Biol Macromol. 2025. PMID: 39832580
-
Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (L-lysine) as antioxidant and antibacterial wound dressing materials.Int J Pharm. 2021 May 15;601:120525. doi: 10.1016/j.ijpharm.2021.120525. Epub 2021 Mar 27. Int J Pharm. 2021. PMID: 33781878
-
Preparation and characterization of electrospun poly(lactic acid)-chitosan core-shell nanofibers with a new solvent system.Int J Biol Macromol. 2019 Oct 1;138:1130-1137. doi: 10.1016/j.ijbiomac.2019.07.053. Epub 2019 Jul 9. Int J Biol Macromol. 2019. PMID: 31299256
-
Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review.Pharmaceutics. 2019 Jul 1;11(7):305. doi: 10.3390/pharmaceutics11070305. Pharmaceutics. 2019. PMID: 31266186 Free PMC article. Review.
-
Core-shell nanofibers as drug delivery systems.Acta Pharm. 2019 Jun 1;69(2):131-153. doi: 10.2478/acph-2019-0014. Acta Pharm. 2019. PMID: 31259723 Review.
Cited by
-
Biobran-loaded core/shell nanofibrous scaffold: a promising wound dressing candidate.RSC Adv. 2024 Feb 7;14(7):4930-4945. doi: 10.1039/d3ra08609g. eCollection 2024 Jan 31. RSC Adv. 2024. PMID: 38327812 Free PMC article.
-
Nanofabrication Techniques for Enhancing Plant-Microbe Interactions in Sustainable Agriculture.Nanomaterials (Basel). 2025 Jul 14;15(14):1086. doi: 10.3390/nano15141086. Nanomaterials (Basel). 2025. PMID: 40711205 Free PMC article. Review.
-
Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers.Polymers (Basel). 2023 Feb 12;15(4):921. doi: 10.3390/polym15040921. Polymers (Basel). 2023. PMID: 36850206 Free PMC article. Review.
-
Preparation Method and Application of Porous Poly(lactic acid) Membranes: A Review.Polymers (Basel). 2024 Jun 28;16(13):1846. doi: 10.3390/polym16131846. Polymers (Basel). 2024. PMID: 39000701 Free PMC article. Review.
-
Evaluation of Polymeric Micro/Nanofibrous Hybrid Scaffolds Prepared via Centrifugal Nozzleless Spinning for Tissue Engineering Applications.Polymers (Basel). 2025 Jan 31;17(3):386. doi: 10.3390/polym17030386. Polymers (Basel). 2025. PMID: 39940588 Free PMC article.
References
-
- Yousefzadeh M., Ghasemkhah F. Handbook of Nanofibers. Springer International Publishing; Berlin, Germany: 2018. Design of Porous, Core-Shell, and Hollow Nanofibers; pp. 1–58.
LinkOut - more resources
Full Text Sources
Miscellaneous

