Highland Barley and Its By-Products Enriched with Phenolic Compounds for Inhibition of Pyrraline Formation by Scavenging α-Dicarbonyl Compounds
- PMID: 34067809
- PMCID: PMC8156036
- DOI: 10.3390/foods10051109
Highland Barley and Its By-Products Enriched with Phenolic Compounds for Inhibition of Pyrraline Formation by Scavenging α-Dicarbonyl Compounds
Abstract
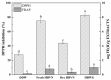
Pyrraline, a typical kind of advanced glycation end product, has been found to contribute to the development of pathologies associated with ageing and diabetes mellitus. In the study, phenolic compounds extracted from highland barley whole grain (HBWG) and vinasse (HBVN) were used to inhibit pyrraline formation in a simulated food. The optimal extraction condition for HBWG and HBVN was using 8 mL of 50% acetone solution at 50 °C for 60 min. The extraction and identification of phenolic compounds from HBWG and HBVN were performed by UPLC-PAD-MS/MS. The inhibitory effects of pyrraline in the simulated food were 52.03% and 49.22% by HBVN and HBWG, respectively. The diphenyl picrylhydrazyl radical- and ferric-reducing ability of plasma assays was used to evaluate the antioxidant activity of the extracts. The main inhibition pathways and molecular mechanism of phenolic compounds on pyrraline regulation were explored by scavenging α-dicarbonyl compounds. The study demonstrated that highland barley and its by-products can potentially be used as a functional food to regulate pyrraline formation during food processing.
Keywords: 3-deoxyglucosone; advanced glycation end products; glyoxal; methylglyoxal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Effects of Highland Barley Bran Extract Rich in Phenolic Acids on the Formation of Nε-Carboxymethyllysine in a Biscuit Model.J Agric Food Chem. 2018 Feb 28;66(8):1916-1922. doi: 10.1021/acs.jafc.7b04957. Epub 2018 Feb 16. J Agric Food Chem. 2018. PMID: 29414239
-
Molecularly imprinted thermosensitive probe based on fluorescent advanced glycation end products to detect α-dicarbonyl compounds and inhibit pyrraline formation.Anal Bioanal Chem. 2023 Aug;415(20):5011-5021. doi: 10.1007/s00216-023-04787-4. Epub 2023 Jun 21. Anal Bioanal Chem. 2023. PMID: 37341783
-
Effect of fatty acids and triglycerides on the formation of lysine-derived advanced glycation end-products in model systems exposed to frying temperature.RSC Adv. 2019 May 14;9(27):15162-15170. doi: 10.1039/c9ra01410a. eCollection 2019 May 14. RSC Adv. 2019. PMID: 35514805 Free PMC article.
-
[Oxidative stress and diabetes mellitus: a possible role of alpha-dicarbonyl compounds in free radical formation].Nihon Ronen Igakkai Zasshi. 1997 Sep;34(9):716-20. Nihon Ronen Igakkai Zasshi. 1997. PMID: 9430981 Review. Japanese.
-
Modulation of 1,2-Dicarbonyl Compounds in Postprandial Responses Mediated by Food Bioactive Components and Mediterranean Diet.Antioxidants (Basel). 2022 Aug 3;11(8):1513. doi: 10.3390/antiox11081513. Antioxidants (Basel). 2022. PMID: 36009232 Free PMC article. Review.
Cited by
-
Fluorescent Molecularly Imprinted Polymers Loaded with Avenanthramides for Inhibition of Advanced Glycation End Products.Polymers (Basel). 2023 Jan 20;15(3):538. doi: 10.3390/polym15030538. Polymers (Basel). 2023. PMID: 36771840 Free PMC article.
References
-
- Maillard L.C. Action des acids amines sur les sucres: Formation des melanoidines par voie methodique. Comptes R. Acad. Sci. 1912;154:66–68.
-
- Yu H.J., Wang J.H., Tang Z., Li X., Yin M.Q., Zhang F., Shu J., Chen W.T., Yang S., Li Z. Integrated glycomics strategy for the evaluation of glycosylation alterations in salivary proteins associated with type 2 diabetes mellitus. RSC Adv. 2020;10:39739–39752. doi: 10.1039/D0RA05466F. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

