Synthesis of Dense 1,2,3-Triazole Polymers Soluble in Common Organic Solvents
- PMID: 34067908
- PMCID: PMC8156623
- DOI: 10.3390/polym13101627
Synthesis of Dense 1,2,3-Triazole Polymers Soluble in Common Organic Solvents
Abstract
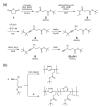
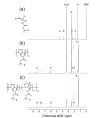
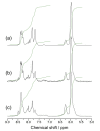
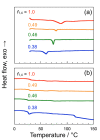
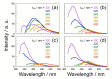
Aiming at synthesis of dense 1,2,3-triazole polymers soluble in common organic solvents, a new 3-azido-1-propyne derivative, i.e., t-butyl 4-azido-5-hexynoate (tBuAH), was synthesized and polymerized by copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and Huisgen cycloaddition (HC). CuAAC polymerization produced poly(tBuAH) composed of 1,4-disubstituted 1,2,3-triazole units (1,4-units), whereas HC polymerization gave poly(tBuAH) composed of 1,4- and 1,5-disubstituted 1,2,3-triazole units (1,4- and 1,5-units). In HC polymerization, the fraction of 1,4-unit (f1,4) decreased with the permittivity of solvent used. Differential scanning calorimetry data indicated that the melting point of poly(tBuAH) increased from 61 to 89 °C with increasing f1,4 from 0.38 to 1.0, indicative of higher crystallinity of poly(tBuAH) composed of 1,4-unit. Preliminary steady-state fluorescence study indicated that all the poly(tBuAH) samples of different f1,4 emitted weak but significant fluorescence in DMF. The maximum of fluorescence band shifted from ca. 350 to ca. 450 nm with varying the excitation wavelength from 300 to 400 nm.
Keywords: 1,2,3-triazole; Huisgen cycloaddition polymerization; copper(I)-catalyzed azide–alkyne cycloaddition polymerization; t-butyl 3-azide-6-hexynoate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synthesis of Dense 1,2,3-Triazole Oligomers Consisting Preferentially of 1,5-Disubstituted Units via Ruthenium(II)-Catalyzed Azide-Alkyne Cycloaddition.Polymers (Basel). 2023 May 5;15(9):2199. doi: 10.3390/polym15092199. Polymers (Basel). 2023. PMID: 37177345 Free PMC article.
-
Emission Properties of Diblock Copolymers Composed of Poly(ethylene glycol) and Dense 1,2,3-Triazole Blocks.Polymers (Basel). 2019 Jun 26;11(7):1086. doi: 10.3390/polym11071086. Polymers (Basel). 2019. PMID: 31247953 Free PMC article.
-
Synthesis of New Thermoresponsive Polymers Possessing the Dense 1,2,3-Triazole Backbone.Langmuir. 2022 May 3;38(17):5156-5165. doi: 10.1021/acs.langmuir.1c02266. Epub 2021 Nov 19. Langmuir. 2022. PMID: 34797074
-
CuAAC-inspired synthesis of 1,2,3-triazole-bridged porphyrin conjugates: an overview.Beilstein J Org Chem. 2023 Mar 22;19:349-379. doi: 10.3762/bjoc.19.29. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 36998309 Free PMC article. Review.
-
Tricks with clicks: modification of peptidomimetic oligomers via copper-catalyzed azide-alkyne [3 + 2] cycloaddition.Chem Soc Rev. 2010 Apr;39(4):1325-37. doi: 10.1039/b901977b. Epub 2010 Mar 4. Chem Soc Rev. 2010. PMID: 20309489 Review.
Cited by
-
Density Function Theory Study on the Energy and Circular Dichroism Spectrum for Methylene-Linked Triazole Diads Depending on the Substitution Position and Conformation.Molecules. 2024 Jun 20;29(12):2931. doi: 10.3390/molecules29122931. Molecules. 2024. PMID: 38930995 Free PMC article.
-
Novel Cu(ii) acidic deep eutectic solvent as an efficient and green multifunctional catalytic solvent system in base-free conditions to synthesize 1,4-disubstituted 1,2,3-triazoles.RSC Adv. 2023 Dec 14;13(51):36403-36415. doi: 10.1039/d3ra06570g. eCollection 2023 Dec 8. RSC Adv. 2023. PMID: 38099257 Free PMC article.
-
Synthesis of Dense 1,2,3-Triazole Oligomers Consisting Preferentially of 1,5-Disubstituted Units via Ruthenium(II)-Catalyzed Azide-Alkyne Cycloaddition.Polymers (Basel). 2023 May 5;15(9):2199. doi: 10.3390/polym15092199. Polymers (Basel). 2023. PMID: 37177345 Free PMC article.
-
Comparison of Proanthocyanidin Content in Rabbiteye Blueberry (Vaccinium virgatum Aiton) Leaves and the Promotion of Apoptosis against HL-60 Promyelocytic Leukemia Cells Using 'Kunisato 35 Gou' Leaf Extract.Plants (Basel). 2023 Feb 19;12(4):948. doi: 10.3390/plants12040948. Plants (Basel). 2023. PMID: 36840296 Free PMC article.
-
Synthesis of a New Polyanion Possessing Dense 1,2,3-Triazole Backbone.Polymers (Basel). 2021 May 17;13(10):1614. doi: 10.3390/polym13101614. Polymers (Basel). 2021. PMID: 34067589 Free PMC article.
References
-
- Huisgen R., Grashey R., Aufderhaar E., Kunz R. 1,3-Dipolar cycloadditions. XIII. Additions of nitrilimines to oximes, azines, and other CN double bonds. Chem. Ber. 1965;98:642–649. doi: 10.1002/cber.19650980245. - DOI
-
- Huisgen R. The concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates. J. Org. Chem. 1976;41:403–419. doi: 10.1021/jo00865a001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

