Structural and Functional Peculiarities of Cytoplasmic Tropomyosin Isoforms, the Products of TPM1 and TPM4 Genes
- PMID: 34067970
- PMCID: PMC8152229
- DOI: 10.3390/ijms22105141
Structural and Functional Peculiarities of Cytoplasmic Tropomyosin Isoforms, the Products of TPM1 and TPM4 Genes
Abstract
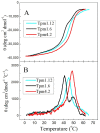
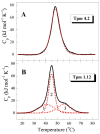
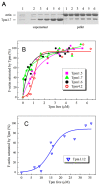
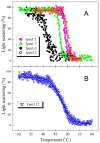
Tropomyosin (Tpm) is one of the major protein partners of actin. Tpm molecules are α-helical coiled-coil protein dimers forming a continuous head-to-tail polymer along the actin filament. Human cells produce a large number of Tpm isoforms that are thought to play a significant role in determining actin cytoskeletal functions. Even though the role of these Tpm isoforms in different non-muscle cells is more or less studied in many laboratories, little is known about their structural and functional properties. In the present work, we have applied various methods to investigate the properties of five cytoplasmic Tpm isoforms (Tpm1.5, Tpm 1.6, Tpm1.7, Tpm1.12, and Tpm 4.2), which are the products of two different genes, TPM1 and TPM4, and also significantly differ by alternatively spliced exons: N-terminal exons 1a2b or 1b, internal exons 6a or 6b, and C-terminal exons 9a, 9c or 9d. Our results demonstrate that structural and functional properties of these Tpm isoforms are quite different depending on sequence variations in alternatively spliced regions of their molecules. The revealed differences can be important in further studies to explain why various Tpm isoforms interact uniquely with actin filaments, thus playing an important role in the organization and dynamics of the cytoskeleton.
Keywords: actin filaments; circular dichroism; cytoplasmic isoforms; differential scanning calorimetry; tropomyosin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Thermal unfolding of various human non-muscle isoforms of tropomyosin.Biochem Biophys Res Commun. 2019 Jun 30;514(3):613-617. doi: 10.1016/j.bbrc.2019.05.008. Epub 2019 May 7. Biochem Biophys Res Commun. 2019. PMID: 31072616
-
The impact of tropomyosins on actin filament assembly is isoform specific.Bioarchitecture. 2016 Jul 3;6(4):61-75. doi: 10.1080/19490992.2016.1201619. Epub 2016 Jul 15. Bioarchitecture. 2016. PMID: 27420374 Free PMC article.
-
Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9.Int J Mol Sci. 2024 Jun 22;25(13):6873. doi: 10.3390/ijms25136873. Int J Mol Sci. 2024. PMID: 38999987 Free PMC article.
-
Tropomyosin - master regulator of actin filament function in the cytoskeleton.J Cell Sci. 2015 Aug 15;128(16):2965-74. doi: 10.1242/jcs.172502. Epub 2015 Aug 3. J Cell Sci. 2015. PMID: 26240174 Review.
-
Vertebrate tropomyosin: distribution, properties and function.J Muscle Res Cell Motil. 2001;22(1):5-49. doi: 10.1023/a:1010303732441. J Muscle Res Cell Motil. 2001. PMID: 11563548 Review.
Cited by
-
Genome-wide analysis of dysregulated RNA-binding proteins and alternative splicing genes in keloid.Front Genet. 2023 Jan 26;14:1118999. doi: 10.3389/fgene.2023.1118999. eCollection 2023. Front Genet. 2023. PMID: 36777722 Free PMC article.
-
Comparison of differentially expressed genes in longissimus dorsi muscle of Diannan small ears, Wujin and landrace pigs using RNA-seq.Front Vet Sci. 2024 Jan 5;10:1296208. doi: 10.3389/fvets.2023.1296208. eCollection 2023. Front Vet Sci. 2024. PMID: 38249550 Free PMC article.
-
Distinct actin-tropomyosin cofilament populations drive the functional diversification of cytoskeletal myosin motor complexes.iScience. 2022 May 30;25(7):104484. doi: 10.1016/j.isci.2022.104484. eCollection 2022 Jul 15. iScience. 2022. PMID: 35720262 Free PMC article.
-
CYP1B1 Knockout in a Bovine Hepatocyte-like Cell Line (BFH12) Unveils Its Role in Liver Homeostasis and Aflatoxin B1-Induced Hepatotoxicity.Toxins (Basel). 2025 Jun 10;17(6):294. doi: 10.3390/toxins17060294. Toxins (Basel). 2025. PMID: 40559872 Free PMC article.
-
Nanopore sequencing reveals full-length Tropomyosin 1 isoforms and their regulation by RNA-binding proteins during rat heart development.J Cell Mol Med. 2021 Sep;25(17):8352-8362. doi: 10.1111/jcmm.16795. Epub 2021 Jul 24. J Cell Mol Med. 2021. PMID: 34302435 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

