Combined Impact of Inflammation and Pharmacogenomic Variants on Voriconazole Trough Concentrations: A Meta-Analysis of Individual Data
- PMID: 34068031
- PMCID: PMC8152514
- DOI: 10.3390/jcm10102089
Combined Impact of Inflammation and Pharmacogenomic Variants on Voriconazole Trough Concentrations: A Meta-Analysis of Individual Data
Abstract
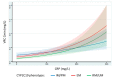
Few studies have simultaneously investigated the impact of inflammation and genetic polymorphisms of cytochromes P450 2C19 and 3A4 on voriconazole trough concentrations. We aimed to define the respective impact of inflammation and genetic polymorphisms on voriconazole exposure by performing individual data meta-analyses. A systematic literature review was conducted using PubMed to identify studies focusing on voriconazole therapeutic drug monitoring with data of both inflammation (assessed by C-reactive protein level) and the pharmacogenomics of cytochromes P450. Individual patient data were collected and analyzed in a mixed-effect model. In total, 203 patients and 754 voriconazole trough concentrations from six studies were included. Voriconazole trough concentrations were independently influenced by age, dose, C-reactive protein level, and both cytochrome P450 2C19 and 3A4 genotype, considered individually or through a combined genetic score. An increase in the C-reactive protein of 10, 50, or 100 mg/L was associated with an increased voriconazole trough concentration of 6, 35, or 82%, respectively. The inhibitory effect of inflammation appeared to be less important for patients with loss-of-function polymorphisms for cytochrome P450 2C19. Voriconazole exposure is influenced by age, inflammatory status, and the genotypes of both cytochromes P450 2C19 and 3A4, suggesting that all these determinants need to be considered in approaches of personalization of voriconazole treatment.
Keywords: inflammation; personalized treatment; pharmacogenomics; therapeutic drug monitoring; voriconazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tissot F., Agrawal S., Pagano L., Petrikkos G., Groll A.H., Skiada A., Lass-Flörl C., Calandra T., Viscoli C., Herbrecht R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433–444. doi: 10.3324/haematol.2016.152900. - DOI - PMC - PubMed
-
- Patterson T.F., Thompson G.R., Denning D.W., Fishman J.A., Hadley S., Herbrecht R., Kontoyiannis D.P., Marr K.A., Morrison V.A., Nguyen M.H., et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. - DOI - PMC - PubMed
-
- van de Peppel R.J., von dem Borne P.A., le Cessie S., de Boer M.G.J. A new time-dependent approach for assessment of the impact of invasive aspergillosis shows effect on short- but not on long-term survival of patients with AML or high-risk MDS. Bone Marrow Transpl. 2017;52:883–888. doi: 10.1038/bmt.2017.71. - DOI - PubMed
-
- Park W.B., Kim N.-H., Kim K.-H., Lee S.H., Nam W.-S., Yoon S.H., Song K.-H., Choe P.G., Kim N.J., Jang I.-J., et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: A randomized controlled trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;55:1080–1087. doi: 10.1093/cid/cis599. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

