Multi-Functional MPT Protein as a Therapeutic Agent against Mycobacterium tuberculosis
- PMID: 34068051
- PMCID: PMC8152475
- DOI: 10.3390/biomedicines9050545
Multi-Functional MPT Protein as a Therapeutic Agent against Mycobacterium tuberculosis
Abstract
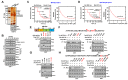
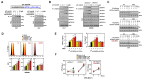
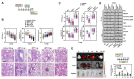
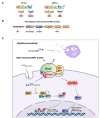
Mycobacterium tuberculosis (MTB), the causative agent of tuberculosis (TB), avoids the host immune system through its virulence factors. MPT63 and MPT64 are the virulence factors secreted by MTB which regulate host proteins for the survival and proliferation of MTB in the host. Here, we found that MPT63 bound directly with TBK1 and p47phox, whereas MPT64 interacted with TBK1 and HK2. We constructed a MPT63/64-derived multifunctional recombinant protein (rMPT) that was able to interact with TBK1, p47phox, or HK2. rMPT was shown to regulate IFN-β levels and increase inflammation and concentration of reactive oxygen species (ROS), while targeting macrophages and killing MTB, both in vitro and in vivo. Furthermore, the identification of the role of rMPT against MTB was achieved via vaccination in a mouse model. Taken together, we here present rMPT, which, by regulating important immune signaling systems, can be considered an effective vaccine or therapeutic agent against MTB.
Keywords: MPT peptide; Mycobacterium tuberculosis; TBK1; hexokinase 2; macrophages; p47phox.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
MPT-51/CpG DNA vaccine protects mice against Mycobacterium tuberculosis.Vaccine. 2009 Jul 16;27(33):4402-7. doi: 10.1016/j.vaccine.2009.05.049. Epub 2009 Jun 2. Vaccine. 2009. PMID: 19500525
-
Mycobacterium tuberculosis Infection-Driven Foamy Macrophages and Their Implications in Tuberculosis Control as Targets for Host-Directed Therapy.Front Immunol. 2020 May 12;11:910. doi: 10.3389/fimmu.2020.00910. eCollection 2020. Front Immunol. 2020. PMID: 32477367 Free PMC article. Review.
-
The immunogenicity of Mycobacterium paratuberculosis 85B antigen.Med Microbiol Immunol. 2002 Mar;190(4):179-87. doi: 10.1007/s00430-001-0104-z. Med Microbiol Immunol. 2002. PMID: 12005331
-
Conformational Switch Driven Membrane Pore Formation by Mycobacterium Secretory Protein MPT63 Induces Macrophage Cell Death.ACS Chem Biol. 2019 Jul 19;14(7):1601-1610. doi: 10.1021/acschembio.9b00327. Epub 2019 Jul 8. ACS Chem Biol. 2019. PMID: 31241303
-
Genome wide approaches discover novel Mycobacterium tuberculosis antigens as correlates of infection, disease, immunity and targets for vaccination.Semin Immunol. 2018 Oct;39:88-101. doi: 10.1016/j.smim.2018.07.001. Epub 2018 Jul 7. Semin Immunol. 2018. PMID: 30327124 Review.
Cited by
-
Expression and epitope prediction of MPT64 recombinant proteins from clinical isolates of Mycobacterium tuberculosis as immunoserodiagnostic candidates.Vet World. 2022 Oct;15(10):2376-2383. doi: 10.14202/vetworld.2022.2376-2383. Epub 2022 Oct 8. Vet World. 2022. PMID: 36425128 Free PMC article.
-
Immunoinformatics analysis of the proteins MPT83 and MPT51 to design a possible chimeric vaccine against Mycobacterium tuberculosis.Braz J Microbiol. 2025 Aug 6. doi: 10.1007/s42770-025-01755-1. Online ahead of print. Braz J Microbiol. 2025. PMID: 40768028
-
Colon-Targeted eNAMPT-Specific Peptide Systems for Treatment of DSS-Induced Acute and Chronic Colitis in Mouse.Antioxidants (Basel). 2022 Nov 30;11(12):2376. doi: 10.3390/antiox11122376. Antioxidants (Basel). 2022. PMID: 36552583 Free PMC article.
-
Toward Mycobacterium tuberculosis Virulence Inhibition: Beyond Cell Wall.Microorganisms. 2024 Dec 26;13(1):21. doi: 10.3390/microorganisms13010021. Microorganisms. 2024. PMID: 39858789 Free PMC article. Review.
-
Dual alarmin-receptor-specific targeting peptide systems for treatment of sepsis.Acta Pharm Sin B. 2024 Dec;14(12):5451-5463. doi: 10.1016/j.apsb.2024.08.015. Epub 2024 Aug 19. Acta Pharm Sin B. 2024. PMID: 39807314 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

