Principles and Practical Considerations for the Analysis of Disease-Associated Alternative Splicing Events Using the Gateway Cloning-Based Minigene Vectors pDESTsplice and pSpliceExpress
- PMID: 34068052
- PMCID: PMC8152502
- DOI: 10.3390/ijms22105154
Principles and Practical Considerations for the Analysis of Disease-Associated Alternative Splicing Events Using the Gateway Cloning-Based Minigene Vectors pDESTsplice and pSpliceExpress
Abstract
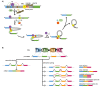
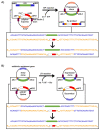
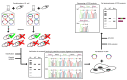
Splicing is an important RNA processing step. Genetic variations can alter the splicing process and thereby contribute to the development of various diseases. Alterations of the splicing pattern can be examined by gene expression analyses, by computational tools for predicting the effects of genetic variants on splicing, and by splicing reporter minigene assays for studying alternative splicing events under defined conditions. The minigene assay is based on transient transfection of cells with a vector containing a genomic region of interest cloned between two constitutive exons. Cloning can be accomplished by the use of restriction enzymes or by site-specific recombination using Gateway cloning. The vectors pDESTsplice and pSpliceExpress represent two minigene systems based on Gateway cloning, which are available through the Addgene plasmid repository. In this review, we describe the features of these two splicing reporter minigene systems. Moreover, we provide an overview of studies in which determinants of alternative splicing were investigated by using pDESTsplice or pSpliceExpress. The studies were reviewed with regard to the investigated splicing regulatory events and the experimental strategy to construct and perform a splicing reporter minigene assay. We further elaborate on how analyses on the regulation of RNA splicing offer promising prospects for gaining important insights into disease mechanisms.
Keywords: RNA processing; alternative splicing; gateway cloning; pDESTsplice; pSpliceExpress; splicing regulation; splicing reporter minigene assay.
Conflict of interest statement
The authors declare no conflict of interest in relation to this article.
Figures
Similar articles
-
Rapid generation of splicing reporters with pSpliceExpress.Gene. 2008 Dec 31;427(1-2):104-10. doi: 10.1016/j.gene.2008.09.021. Epub 2008 Oct 1. Gene. 2008. PMID: 18930792 Free PMC article.
-
Use of splicing reporter minigene assay to evaluate the effect on splicing of unclassified genetic variants.Methods Mol Biol. 2010;653:249-57. doi: 10.1007/978-1-60761-759-4_15. Methods Mol Biol. 2010. PMID: 20721748 Review.
-
Homologous recombination-mediated cloning and manipulation of genomic DNA regions using Gateway and recombineering systems.BMC Biotechnol. 2008 Nov 17;8:88. doi: 10.1186/1472-6750-8-88. BMC Biotechnol. 2008. PMID: 19014699 Free PMC article.
-
Minigene reporter for identification and analysis of cis elements and trans factors affecting pre-mRNA splicing.Biotechniques. 2006 Aug;41(2):177-81. doi: 10.2144/000112208. Biotechniques. 2006. PMID: 16925019
-
Diagnostics of pathogenic splicing mutations: does bioinformatics cover all bases?Front Biosci. 2008 May 1;13:3252-72. doi: 10.2741/2924. Front Biosci. 2008. PMID: 18508431 Review.
Cited by
-
Molecular Basis of Unequal Alternative Splicing of Human SCD5 and Its Alteration by Natural Genetic Variations.Int J Mol Sci. 2023 Mar 30;24(7):6517. doi: 10.3390/ijms24076517. Int J Mol Sci. 2023. PMID: 37047490 Free PMC article.
-
Functional and structural analysis of a novel splice site HMBS variant in a Chinese AIP patient.Front Genet. 2023 Dec 19;14:1333111. doi: 10.3389/fgene.2023.1333111. eCollection 2023. Front Genet. 2023. PMID: 38192441 Free PMC article.
-
A Novel Mutation c.3392G>T of COL2A1 Causes Spondyloepiphyseal Dysplasia Congenital by Affecting Pre-mRNA Splicing.Front Genet. 2022 Apr 5;13:827560. doi: 10.3389/fgene.2022.827560. eCollection 2022. Front Genet. 2022. PMID: 35692839 Free PMC article.
-
Integrating next-generation sequencing and artificial intelligence for the identification and validation of pathogenic variants in colorectal cancer.Front Oncol. 2025 May 19;15:1568205. doi: 10.3389/fonc.2025.1568205. eCollection 2025. Front Oncol. 2025. PMID: 40458721 Free PMC article.
-
New Variants of the Cytochrome P450 2R1 (CYP2R1) Gene in Individuals with Severe Vitamin D-Activating Enzyme 25(OH)D Deficiency.Biomolecules. 2021 Dec 12;11(12):1867. doi: 10.3390/biom11121867. Biomolecules. 2021. PMID: 34944511 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

