Crosstalk between Peroxisomal Activities and Nrf2 Signaling in Porcine Embryos
- PMID: 34068072
- PMCID: PMC8152488
- DOI: 10.3390/antiox10050771
Crosstalk between Peroxisomal Activities and Nrf2 Signaling in Porcine Embryos
Abstract
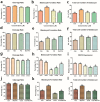
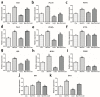
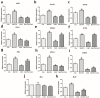
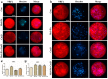
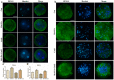
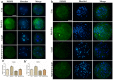
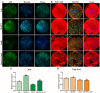
Melatonin and phytanic acid (PA) are known to be involved in lipid metabolism and β-oxidation, in which peroxisomal activities also significantly participate. In addition, other studies have reported that the nuclear factor-erythroid-derived 2-like 2 (Nrf2 or NFE2L2) signaling pathway mediates lipid metabolism and its subsequent cascades. As these mechanisms are partially involved in porcine oocytes or embryonic development, we hypothesized that the factors governing these mechanisms could be interconnected. Therefore, we aimed to investigate possible crosstalk between peroxisomal activities and Nrf2 signaling in porcine embryos following melatonin and PA treatment. Porcine embryos were cultured for seven days after parthenogenetic activation, and subsequently treated with melatonin and PA, or injected with Pex19-targeted siRNAs. Real-time PCR, immunocytochemistry, and BODIPY staining were used to evaluate peroxisomal activities, Nrf2 signaling, and subsequent lipid metabolism. We found that melatonin/PA treatment enhanced embryonic development, whereas injection with Pex19-targeted siRNAs had the opposite effect. Moreover, melatonin/PA treatment upregulated peroxisomal activities, Nrf2 signaling, lipid metabolism, and mitochondrial membrane potentials, whereas most of these mechanisms were downregulated by Pex19-targeted siRNAs. Therefore, we suggest that there is a connection between the action of melatonin and PA and the Nrf2 signaling pathway and peroxisomal activities, which positively influences porcine embryonic development.
Keywords: IVC; Nrf2 signaling; lipid metabolism; melatonin; phytanic acid; porcine embryos.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

