Effect of Hydroxychloroquine on QTc in Patients Diagnosed with COVID-19: A Systematic Review and Meta-Analysis
- PMID: 34068104
- PMCID: PMC8152730
- DOI: 10.3390/jcdd8050055
Effect of Hydroxychloroquine on QTc in Patients Diagnosed with COVID-19: A Systematic Review and Meta-Analysis
Abstract
Background: Hydroxychloroquine or chloroquine with or without the concomitant use of azithromycin have been widely used to treat patients with SARS-CoV-2 infection, based on early in vitro studies, despite their potential to prolong the QTc interval of patients.
Objective: This is a systematic review and metanalysis designed to assess the effect of hydroxychloroquine with or without the addition of azithromycin on the QTc of hospitalized patients with COVID-19.
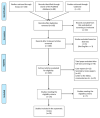
Materials and methods: PubMed, Scopus, Cochrane and MedRxiv databases were reviewed. A random effect model meta-analysis was used, and I-square was used to assess the heterogeneity. The prespecified endpoints were ΔQTc, QTc prolongation > 500 ms and ΔQTc > 60 ms.
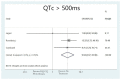
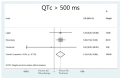
Results: A total of 18 studies and 7179 patients met the inclusion criteria and were included in this systematic review and meta-analysis. The use of hydroxychloroquine with or without the addition of azithromycin was associated with increased QTc when used as part of the management of patients with SARS-CoV-2 infection. The combination therapy with hydroxychloroquine plus azithromycin was also associated with statistically significant increases in QTc. Moreover, the use of hydroxychloroquine alone, azithromycin alone, or the combination of the two was associated with increased numbers of patients that developed QTc prolongation > 500 ms.
Conclusion: This systematic review and metanalysis revealed that the use of hydroxychloroquine alone or in conjunction with azithromycin was linked to an increase in the QTc interval of hospitalized patients with SARS-CoV-2 infection that received these agents.
Keywords: COVID-19; QTc; QTc prolongation; SARS-CoV-2; chloroquine; coronavirus; hydroxychloroquine; torsades de pointes.
Conflict of interest statement
The authors have no potential conflict of interest regarding the research or publication of this manuscript.
Figures




Similar articles
-
Hydroxychloroquine and QTc prolongation in patients with COVID-19: A systematic review and meta-analysis.Indian Pacing Electrophysiol J. 2021 Jan-Feb;21(1):36-43. doi: 10.1016/j.ipej.2020.10.002. Epub 2020 Oct 16. Indian Pacing Electrophysiol J. 2021. PMID: 33075484 Free PMC article. Review.
-
QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis.Pharmacoepidemiol Drug Saf. 2021 Jun;30(6):694-706. doi: 10.1002/pds.5234. Epub 2021 Apr 3. Pharmacoepidemiol Drug Saf. 2021. PMID: 33772933 Free PMC article.
-
Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring.J Am Heart Assoc. 2020 Jun 16;9(12):e017144. doi: 10.1161/JAHA.120.017144. Epub 2020 May 28. J Am Heart Assoc. 2020. PMID: 32463348 Free PMC article.
-
Hydroxychloroquine/Azithromycin Therapy and QT Prolongation in Hospitalized Patients With COVID-19.JACC Clin Electrophysiol. 2021 Jan;7(1):16-25. doi: 10.1016/j.jacep.2020.07.016. Epub 2020 Aug 5. JACC Clin Electrophysiol. 2021. PMID: 33478708 Free PMC article.
-
Cardiac Corrected QT Interval Changes Among Patients Treated for COVID-19 Infection During the Early Phase of the Pandemic.JAMA Netw Open. 2021 Apr 1;4(4):e216842. doi: 10.1001/jamanetworkopen.2021.6842. JAMA Netw Open. 2021. PMID: 33890991 Free PMC article.
References
-
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Maddeb B., Mailhe M., Doubier B., Courjon J., Giordanengo V., Vieira V.E., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

