Biocompatible Ir(III) Complexes as Oxygen Sensors for Phosphorescence Lifetime Imaging
- PMID: 34068190
- PMCID: PMC8153025
- DOI: 10.3390/molecules26102898
Biocompatible Ir(III) Complexes as Oxygen Sensors for Phosphorescence Lifetime Imaging
Abstract
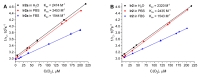
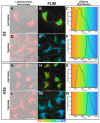
Synthesis of biocompatible near infrared phosphorescent complexes and their application in bioimaging as triplet oxygen sensors in live systems are still challenging areas of organometallic chemistry. We have designed and synthetized four novel iridium [Ir(N^C)2(N^N)]+ complexes (N^C-benzothienyl-phenanthridine based cyclometalated ligand; N^N-pyridin-phenanthroimidazol diimine chelate), decorated with oligo(ethylene glycol) groups to impart these emitters' solubility in aqueous media, biocompatibility, and to shield them from interaction with bio-environment. These substances were fully characterized using NMR spectroscopy and ESI mass-spectrometry. The complexes exhibited excitation close to the biological "window of transparency", NIR emission at 730 nm, and quantum yields up to 12% in water. The compounds with higher degree of the chromophore shielding possess low toxicity, bleaching stability, absence of sensitivity to variations of pH, serum, and complex concentrations. The properties of these probes as oxygen sensors for biological systems have been studied by using phosphorescence lifetime imaging experiments in different cell cultures. The results showed essential lifetime response onto variations in oxygen concentration (2.0-2.3 μs under normoxia and 2.8-3.0 μs under hypoxia conditions) in complete agreement with the calibration curves obtained "in cuvette". The data obtained indicate that these emitters can be used as semi-quantitative oxygen sensors in biological systems.
Keywords: Ir(III) complexes; NIR emitters; oxygen sensing; phosphorescence lifetime imaging.
Conflict of interest statement
Author Vladislav Shcheslavskiy is employed by the company Becker&Hickl GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Sanderson T.H., Reynolds C.A., Kumar R., Przyklenk K., Hüttemann M. Molecular mechanisms of ischemia–reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

