Estrogen Receptor-α Suppresses Liver Carcinogenesis and Establishes Sex-Specific Gene Expression
- PMID: 34068249
- PMCID: PMC8153146
- DOI: 10.3390/cancers13102355
Estrogen Receptor-α Suppresses Liver Carcinogenesis and Establishes Sex-Specific Gene Expression
Abstract
Estrogen protects females from hepatocellular carcinoma (HCC). To determine whether this protection is mediated by classic estrogen receptors, we tested HCC susceptibility in estrogen receptor-deficient mice. In contrast to a previous study, we found that diethylnitrosamine induces hepatocarcinogenesis to a significantly greater extent when females lack Esr1, which encodes Estrogen Receptor-α. Relative to wild-type littermates, Esr1 knockout females developed 9-fold more tumors. Deficiency of Esr2, which encodes Estrogen Receptor-β, did not affect liver carcinogenesis in females. Using microarrays and QPCR to examine estrogen receptor effects on hepatic gene expression patterns, we found that germline Esr1 deficiency resulted in the masculinization of gene expression in the female liver. Six of the most dysregulated genes have previously been implicated in HCC. In contrast, Esr1 deletion specifically in hepatocytes of Esr1 conditional null female mice (in which Cre was expressed from the albumin promoter) resulted in the maintenance of female-specific liver gene expression. Wild-type adult females lacking ovarian estrogen due to ovariectomy, which is known to make females susceptible to HCC, also maintained female-specific expression in the liver of females. These studies indicate that Esr1 mediates liver cancer risk, and its control of sex-specific liver gene expression involves cells other than hepatocytes.
Keywords: estrogen receptor; gene expression; hepatocarcinogenesis; liver cancer; ovariectomy; sexual dimorphism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


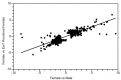
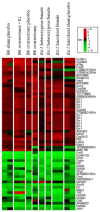

Comment in
-
Sex-specific changes in the expression of ER-alpha and androgen receptor with increasing tumor grade in patients with hepatocellular carcinoma.Hum Cell. 2022 May;35(3):948-951. doi: 10.1007/s13577-022-00695-4. Epub 2022 Mar 29. Hum Cell. 2022. PMID: 35349116 No abstract available.
Similar articles
-
Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice.Am J Pathol. 2004 Jun;164(6):1915-24. doi: 10.1016/S0002-9440(10)63752-2. Am J Pathol. 2004. PMID: 15161628 Free PMC article.
-
Aberrant DNA methylation suppresses expression of estrogen receptor 1 (ESR1) in ovarian endometrioma.J Ovarian Res. 2019 Feb 6;12(1):14. doi: 10.1186/s13048-019-0489-1. J Ovarian Res. 2019. PMID: 30728052 Free PMC article.
-
The little mutation suppresses DEN-induced hepatocarcinogenesis in mice and abrogates genetic and hormonal modulation of susceptibility.Carcinogenesis. 2001 Nov;22(11):1853-62. doi: 10.1093/carcin/22.11.1853. Carcinogenesis. 2001. PMID: 11698349
-
Sex hormone-binding globulin suppresses NAFLD-triggered hepatocarcinogenesis after menopause.Carcinogenesis. 2019 Aug 22;40(8):1031-1041. doi: 10.1093/carcin/bgz107. Carcinogenesis. 2019. PMID: 31168625
-
Estrogen and Glycemic Homeostasis: The Fundamental Role of Nuclear Estrogen Receptors ESR1/ESR2 in Glucose Transporter GLUT4 Regulation.Cells. 2021 Jan 7;10(1):99. doi: 10.3390/cells10010099. Cells. 2021. PMID: 33430527 Free PMC article. Review.
Cited by
-
Non-canonical activation of MAPK signaling by the lncRNA ASH1L-AS1-encoded microprotein APPLE through inhibition of PP1/PP2A-mediated ERK1/2 dephosphorylation in hepatocellular carcinoma.J Exp Clin Cancer Res. 2025 Jul 11;44(1):200. doi: 10.1186/s13046-025-03465-w. J Exp Clin Cancer Res. 2025. PMID: 40646641 Free PMC article.
-
Estrogen receptor α suppresses hepatocellular carcinoma by restricting M2 macrophage infiltration through the YAP-CCL2 axis.BMC Cancer. 2025 Mar 27;25(1):550. doi: 10.1186/s12885-025-13676-1. BMC Cancer. 2025. PMID: 40148834 Free PMC article.
-
Elucidating the Anti-Diabetic Mechanisms of Mushroom Chaga (Inonotus obliquus) by Integrating LC-MS, Network Pharmacology, Molecular Docking, and Bioinformatics.Int J Mol Sci. 2025 May 28;26(11):5202. doi: 10.3390/ijms26115202. Int J Mol Sci. 2025. PMID: 40508014 Free PMC article.
-
Intricate roles of estrogen and estrogen receptors in digestive system cancers: a systematic review.Cancer Biol Med. 2024 Oct 30;21(10):898-915. doi: 10.20892/j.issn.2095-3941.2024.0224. Cancer Biol Med. 2024. PMID: 39475214 Free PMC article.
-
Cell-specific AHR-driven differential gene expression in the mouse liver cell following acute TCDD exposure.BMC Genomics. 2024 Aug 28;25(1):809. doi: 10.1186/s12864-024-10730-3. BMC Genomics. 2024. PMID: 39198768 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

