Structural Studies Providing Insights into Production and Conformational Behavior of Amyloid-β Peptide Associated with Alzheimer's Disease Development
- PMID: 34068293
- PMCID: PMC8153327
- DOI: 10.3390/molecules26102897
Structural Studies Providing Insights into Production and Conformational Behavior of Amyloid-β Peptide Associated with Alzheimer's Disease Development
Abstract
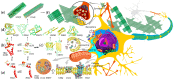
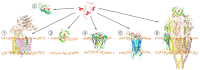
Alzheimer's disease is the most common type of neurodegenerative disease in the world. Genetic evidence strongly suggests that aberrant generation, aggregation, and/or clearance of neurotoxic amyloid-β peptides (Aβ) triggers the disease. Aβ accumulates at the points of contact of neurons in ordered cords and fibrils, forming the so-called senile plaques. Aβ isoforms of different lengths are found in healthy human brains regardless of age and appear to play a role in signaling pathways in the brain and to have neuroprotective properties at low concentrations. In recent years, different substances have been developed targeting Aβ production, aggregation, interaction with other molecules, and clearance, including peptide-based drugs. Aβ is a product of sequential cleavage of the membrane glycoprotein APP (amyloid precursor protein) by β- and γ-secretases. A number of familial mutations causing an early onset of the disease have been identified in the APP, especially in its transmembrane domain. The mutations are reported to influence the production, oligomerization, and conformational behavior of Aβ peptides. This review highlights the results of structural studies of the main proteins involved in Alzheimer's disease pathogenesis and the molecular mechanisms by which perspective therapeutic substances can affect Aβ production and nucleation.
Keywords: Alzheimer’s disease; amyloid precursor protein; amyloid-β peptide; bioactive peptides for therapy and diagnosis; molecular mechanism; neuronal membrane; protein–protein and protein–lipid interactions; structural–dynamical properties; toxic oligomerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

