Pre-Operative Decitabine in Colon Cancer Patients: Analyses on WNT Target Methylation and Expression
- PMID: 34068407
- PMCID: PMC8153633
- DOI: 10.3390/cancers13102357
Pre-Operative Decitabine in Colon Cancer Patients: Analyses on WNT Target Methylation and Expression
Abstract
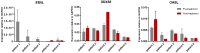
DNA hypermethylation is common in colon cancer. Previously, we have shown that methylation of WNT target genes predicts poor prognosis in stage II colon cancer. The primary objective of this study was to assess whether pre-operative treatment with decitabine can decrease methylation and increase the expression of WNT target genes APCDD1, AXIN2 and DKK1 in colon cancer patients. A clinical study was conducted, investigating these potential effects of decitabine in colon cancer patients (DECO). Patients were treated two times with 25 mg/m2 decitabine before surgery. Methylation and expression of LINE1 and WNT target genes (primary outcome) and expression of endogenous retroviral genes (secondary outcome) were analysed in pre- and post-treatment tumour samples using pyrosequencing and rt-PCR. Ten patients were treated with decitabine and eighteen patients were used as controls. Decitabine treatment only marginally decreased LINE1 methylation. More importantly, no differences in methylation or expression of WNT target or endogenous retroviral genes were observed. Due to the lack of an effect on primary and secondary outcomes, the study was prematurely closed. In conclusion, pre-operative treatment with decitabine is safe, but with the current dosing, the primary objective, increased WNT target gene expression, cannot be achieved.
Keywords: DNA methylation; clinical translation study; colon cancer; decitabine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

