Recombinant Antibody Production Using a Dual-Promoter Single Plasmid System
- PMID: 34068440
- PMCID: PMC8161450
- DOI: 10.3390/antib10020018
Recombinant Antibody Production Using a Dual-Promoter Single Plasmid System
Abstract
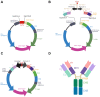
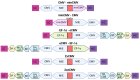
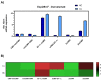
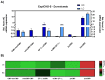
Monoclonal antibodies (mAbs) have demonstrated tremendous effects on the treatment of various disease indications and remain the fastest growing class of therapeutics. Production of recombinant antibodies is performed using mammalian expression systems to facilitate native antibody folding and post-translational modifications. Generally, mAb expression systems utilize co-transfection of heavy chain (hc) and light chain (lc) genes encoded on separate plasmids. In this study, we examine the production of two FDA-approved antibodies using a bidirectional (BiDi) vector encoding both hc and lc with mirrored promoter and enhancer elements on a single plasmid, by analysing the individual hc and lc mRNA expression levels and subsequent quantification of fully-folded IgGs on the protein level. From the assessment of different promoter combinations, we have developed a generic expression vector comprised of mirrored enhanced CMV (eCMV) promoters showing comparable mAb yields to a two-plasmid reference. This study paves the way to facilitate small-scale mAb production by transient cell transfection with a single vector in a cost- and time-efficient manner.
Keywords: antibody production; bidirectional; monoclonal antibodies; promoters; upstream processing.
Conflict of interest statement
J.G. and B.H. are employees of Ferring Pharmaceuticals, while S.C.C., D.F. and J.P.B. are employed by the Technische Universität Darmstadt in frame of a collaboration with Ferring Pharmaceuticals. All authors declare no conflicts of interest.
Figures


Similar articles
-
On the optimal ratio of heavy to light chain genes for efficient recombinant antibody production by CHO cells.Biotechnol Prog. 2005 Jan-Feb;21(1):122-33. doi: 10.1021/bp049780w. Biotechnol Prog. 2005. PMID: 15903249
-
Impact of gene vector design on the control of recombinant monoclonal antibody production by Chinese hamster ovary cells.Biotechnol Prog. 2011 Nov-Dec;27(6):1689-99. doi: 10.1002/btpr.692. Epub 2011 Aug 31. Biotechnol Prog. 2011. PMID: 21882365
-
An IRES-Mediated Tricistronic Vector for Efficient Generation of Stable, High-Level Monoclonal Antibody Producing CHO DG44 Cell Lines.Methods Mol Biol. 2018;1827:335-349. doi: 10.1007/978-1-4939-8648-4_17. Methods Mol Biol. 2018. PMID: 30196505
-
An Efficient Transient Expression System for Enhancing the Generation of Monoclonal Antibodies in 293 Suspension Cells.Curr Pharm Biotechnol. 2017;18(4):351-357. doi: 10.2174/1389201018666170320110545. Curr Pharm Biotechnol. 2017. PMID: 28322160
-
Vector-related stratagems for enhanced monoclonal antibody production in mammalian cells.Biotechnol Adv. 2019 Dec;37(8):107415. doi: 10.1016/j.biotechadv.2019.107415. Epub 2019 Jul 3. Biotechnol Adv. 2019. PMID: 31279066 Review.
Cited by
-
Strategies and Considerations for Improving Recombinant Antibody Production and Quality in Chinese Hamster Ovary Cells.Front Bioeng Biotechnol. 2022 Mar 4;10:856049. doi: 10.3389/fbioe.2022.856049. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35316944 Free PMC article. Review.
-
Construction of an Integration Vector with a Chimeric Signal Peptide for the Expression of Monoclonal Antibodies in Mammalian Cells.Curr Issues Mol Biol. 2024 Dec 22;46(12):14464-14475. doi: 10.3390/cimb46120868. Curr Issues Mol Biol. 2024. PMID: 39727996 Free PMC article.
-
Streamlining the Transition From Yeast Surface Display of Antibody Fragment Immune Libraries to the Production as IgG Format in Mammalian Cells.Front Bioeng Biotechnol. 2022 May 10;10:794389. doi: 10.3389/fbioe.2022.794389. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35620472 Free PMC article.
-
Design, Production, Characterization, and Use of Peptide Antibodies.Antibodies (Basel). 2023 Jan 13;12(1):6. doi: 10.3390/antib12010006. Antibodies (Basel). 2023. PMID: 36648890 Free PMC article.
-
An outlook to sophisticated technologies and novel developments for metabolic regulation in the Saccharomyces cerevisiae expression system.Front Bioeng Biotechnol. 2023 Oct 5;11:1249841. doi: 10.3389/fbioe.2023.1249841. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37869712 Free PMC article. Review.
References
-
- Carvalho L.S., Bravim da Silva O., Carneiro de Almeida G., Davies de Oliveira J., Parachin N.S., Carmo T.S. Production processes for monoclonal antibodies. Intech. 2017 doi: 10.5772/64263. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

