Natural Sulfurs Inhibit LPS-Induced Inflammatory Responses through NF-κB Signaling in CCD-986Sk Skin Fibroblasts
- PMID: 34068523
- PMCID: PMC8151259
- DOI: 10.3390/life11050427
Natural Sulfurs Inhibit LPS-Induced Inflammatory Responses through NF-κB Signaling in CCD-986Sk Skin Fibroblasts
Abstract
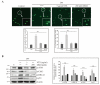
Lipopolysaccharide (LPS)-induced inflammatory response leads to serious damage, up to and including tumorigenesis. Natural mineral sulfur, non-toxic sulfur (NTS), and methylsulfonylmethane (MSM) have anti-inflammatory activity that may inhibit LPS-induced inflammation. We hypothesized that sulfur compounds could inhibit LPS-induced inflammatory responses in CCD-986Sk skin fibroblasts. We used Western blotting and real-time PCR to analyze molecular signaling in treated and untreated cultures. We also used flow cytometry for cell surface receptor analysis, comet assays to evaluate DNA damage, and ELISA-based cytokine detection. LPS induced TLR4 activation and NF-κB signaling via canonical and protein kinase C (PKC)-dependent pathways, while NTS and MSM downregulated that response. NTS and MSM also inhibited LPS-induced nuclear accumulation and binding of NF-κB to proinflammatory cytokines COX-2, IL-1β, and IL-6. Finally, the sulfur compounds suppressed LPS-induced ROS accumulation and DNA damage in CCD-986Sk cells. These results suggest that natural sulfur compounds could be used to treat inflammation and may be useful in the development of cosmetics.
Keywords: CCD-986Sk; LPS; MSM; NF-κB; NTS; ROS; TLR4; anti-inflammatory effect.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

