Semisynthesis of Selenoauraptene
- PMID: 34068532
- PMCID: PMC8126015
- DOI: 10.3390/molecules26092798
Semisynthesis of Selenoauraptene
Abstract
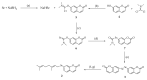
Selenium-containing compounds are gaining more and more interest due to their valuable and promising pharmacological properties, mainly as anticancer and antioxidant agents. Ebselen, the up to now only approved drugs, is well known to possess very good glutathione peroxidase mimicking effects. To date, the most of efforts have been directed to build pure synthetic Se containing molecules, while less attention have been devoted to Se-based semisynthetic products resembling natural compounds like terpenes, polyphenols, and alkaloids. The aim of this short communication is to report the synthesis of the first example of a Se-phenylpropanoids, namely selenoauraptene, containing a selenogeranyl side chain in position 7 of the umbelliferone core. The key step was the Newman-Kwart rearrangement to obtain a selenocarbamate in which the Se atom was directly attached to umbelliferone (replacing its 7-OH function) followed by hydrolysis to get diumbelliferyl diselenide, which was finally easily converted to the desired Se-geranyl derivative in quite a good overall yield (28.5%). The synthesized adduct displayed a greater antioxidant and a radical scavenger in vitro activity than parent auraptene. The procedure we describe herein, to the best of our knowledge for the first time in the literature, represents an easy-to-handle method for the synthesis of a wide array of seleno analogues of naturally occurring biologically active oxyprenylated secondary metabolites.
Keywords: 7-geranylselenocoumarin; Newman-Kwart rearrangement; auraptene; selenium compounds; selenoauraptene; selenoprenylcoumarins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
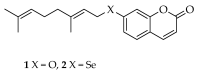
References
-
- Fiorito S., Epifano F., Preziuso F., Taddeo V.A., Genovese S. Biomolecular targets of oxyprenylated phenyl-propanoids and polyketides. Prog. Chem. Org. Nat. Prod. 2019;108:143–205. - PubMed
-
- Derosa G., Maffioli P., Sahebkar A. Auraptene and its role in chronic diseases. Adv. Exp. Meth. Biol. 2016;929:399–407. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

