Bioactive Regeneration Potential of the Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-Lactide-Co-Glycolide Biomaterial in Maxillofacial Reconstructive Surgery: An In Vivo Preliminary Study
- PMID: 34068558
- PMCID: PMC8126161
- DOI: 10.3390/ma14092461
Bioactive Regeneration Potential of the Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-Lactide-Co-Glycolide Biomaterial in Maxillofacial Reconstructive Surgery: An In Vivo Preliminary Study
Abstract
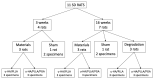
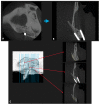
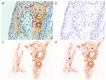
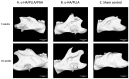
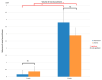
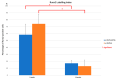
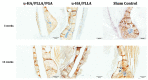
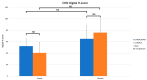
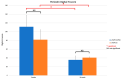
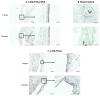
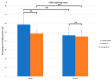
Uncalcined/unsintered hydroxyapatite (HA) and poly-l-lactide-co-glycolide (u-HA/PLLA/PGA) are novel bioresorbable bioactive materials with bone regeneration characteristics and have been used to treat mandibular defects in a rat model. However, the bone regenerative interaction with the periosteum, the inflammatory response, and the degradation of this material have not been examined. In this study, we used a rat mandible model to compare the above features in u-HA/PLLA/PGA and uncalcined/unsintered HA and poly-l-lactic acid (u-HA/PLLA). We divided 11 male Sprague-Dawley rats into 3- and 16-week groups. In each group, we assessed the characteristics of a u-HA/PLLA/PGA sheet covering the right mandibular angle and a u-HA/PLLA sheet covering the left mandibular angle in three rats each, and one rat was used as a sham control. The remaining three rats in the 16-week group were used for a degradation assessment and received both sheets of material as in the material assessment subgroup. At 3 and 16 weeks after surgery, the rats were sacrificed, and mandible specimens were subjected to micro-computed tomography, histological analysis, and immunohistochemical staining. The results indicated that the interaction between the periosteum and u-HA/PLLA/PGA material produced significantly more new bone regeneration with a lower inflammatory response and a faster resorption rate compared to u-HA/PLLA alone. These findings may indicate that this new biomaterial has ideal potential in treating maxillofacial defects of the midface and orbital regions.
Keywords: CD68; Runx2; bone regeneration; osteocalcin; osteoconductivity; periostin; poly-l-lactic acid; poly-l-lactide-co-glycolide; uncalcined/unsintered hydroxyapatite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Narrative Review of u-HA/PLLA, a Bioactive Resorbable Reconstruction Material: Applications in Oral and Maxillofacial Surgery.Materials (Basel). 2021 Dec 26;15(1):150. doi: 10.3390/ma15010150. Materials (Basel). 2021. PMID: 35009297 Free PMC article. Review.
-
Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study.Materials (Basel). 2023 Nov 27;16(23):7379. doi: 10.3390/ma16237379. Materials (Basel). 2023. PMID: 38068124 Free PMC article.
-
Bone Regeneration Capacity of Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-lactide-co-glycolide Sheet in Maxillofacial Surgery: An In Vivo Study.Nanomaterials (Basel). 2020 Dec 24;11(1):22. doi: 10.3390/nano11010022. Nanomaterials (Basel). 2020. PMID: 33374294 Free PMC article.
-
Bone Regeneration Potential of Uncalcined and Unsintered Hydroxyapatite/Poly l-lactide Bioactive/Osteoconductive Sheet Used for Maxillofacial Reconstructive Surgery: An In Vivo Study.Materials (Basel). 2019 Sep 11;12(18):2931. doi: 10.3390/ma12182931. Materials (Basel). 2019. PMID: 31514283 Free PMC article.
-
Navigation-Assisted Orbital Trauma Reconstruction Using a Bioactive Osteoconductive/Bioresorbable u-HA/PLLA System.J Maxillofac Oral Surg. 2019 Sep;18(3):329-338. doi: 10.1007/s12663-019-01207-y. Epub 2019 Mar 21. J Maxillofac Oral Surg. 2019. PMID: 31371870 Free PMC article. Review.
Cited by
-
Comparative In Vitro Dissolution Assessment of Calcined and Uncalcined Hydroxyapatite Using Differences in Bioresorbability and Biomineralization.Int J Mol Sci. 2024 Jan 3;25(1):621. doi: 10.3390/ijms25010621. Int J Mol Sci. 2024. PMID: 38203791 Free PMC article.
-
A Narrative Review of u-HA/PLLA, a Bioactive Resorbable Reconstruction Material: Applications in Oral and Maxillofacial Surgery.Materials (Basel). 2021 Dec 26;15(1):150. doi: 10.3390/ma15010150. Materials (Basel). 2021. PMID: 35009297 Free PMC article. Review.
-
Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study.Materials (Basel). 2023 Nov 27;16(23):7379. doi: 10.3390/ma16237379. Materials (Basel). 2023. PMID: 38068124 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

