Hot Electrons in TiO2-Noble Metal Nano-Heterojunctions: Fundamental Science and Applications in Photocatalysis
- PMID: 34068571
- PMCID: PMC8151081
- DOI: 10.3390/nano11051249
Hot Electrons in TiO2-Noble Metal Nano-Heterojunctions: Fundamental Science and Applications in Photocatalysis
Abstract
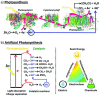
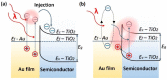
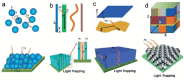
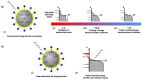
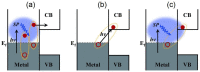
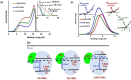
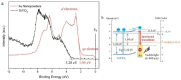
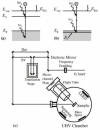
Plasmonic photocatalysis enables innovation by harnessing photonic energy across a broad swathe of the solar spectrum to drive chemical reactions. This review provides a comprehensive summary of the latest developments and issues for advanced research in plasmonic hot electron driven photocatalytic technologies focusing on TiO2-noble metal nanoparticle heterojunctions. In-depth discussions on fundamental hot electron phenomena in plasmonic photocatalysis is the focal point of this review. We summarize hot electron dynamics, elaborate on techniques to probe and measure said phenomena, and provide perspective on potential applications-photocatalytic degradation of organic pollutants, CO2 photoreduction, and photoelectrochemical water splitting-that benefit from this technology. A contentious and hitherto unexplained phenomenon is the wavelength dependence of plasmonic photocatalysis. Many published reports on noble metal-metal oxide nanostructures show action spectra where quantum yields closely follow the absorption corresponding to higher energy interband transitions, while an equal number also show quantum efficiencies that follow the optical response corresponding to the localized surface plasmon resonance (LSPR). We have provided a working hypothesis for the first time to reconcile these contradictory results and explain why photocatalytic action in certain plasmonic systems is mediated by interband transitions and in others by hot electrons produced by the decay of particle plasmons.
Keywords: Schottky barrier; TiO2; charge transfer; hot electron; nanoparticles; optical resonances; oxide interfaces; photoreduction; plasmon; solar energy conversion.
Conflict of interest statement
There are no conflict of interest to declare.
Figures





Similar articles
-
Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis.Small. 2022 Jan;18(1):e2101638. doi: 10.1002/smll.202101638. Epub 2021 Aug 15. Small. 2022. PMID: 34396695 Review.
-
Hot Hole Collection and Photoelectrochemical CO2 Reduction with Plasmonic Au/p-GaN Photocathodes.Nano Lett. 2018 Apr 11;18(4):2545-2550. doi: 10.1021/acs.nanolett.8b00241. Epub 2018 Mar 15. Nano Lett. 2018. PMID: 29522350
-
Plasmonic hot carrier-driven oxygen evolution reaction on Au nanoparticles/TiO2 nanotube arrays.Nanoscale. 2018 Dec 21;10(47):22180-22188. doi: 10.1039/c8nr05144e. Epub 2018 Nov 28. Nanoscale. 2018. PMID: 30484456
-
Harvesting Hot Holes in Plasmon-Coupled Ultrathin Photoanodes for High-Performance Photoelectrochemical Water Splitting.ACS Appl Mater Interfaces. 2021 Sep 15;13(36):42741-42752. doi: 10.1021/acsami.1c10698. Epub 2021 Sep 3. ACS Appl Mater Interfaces. 2021. PMID: 34476945
-
Full-spectrum plasmonic semiconductors for photocatalysis.Mater Horiz. 2024 Nov 11;11(22):5470-5498. doi: 10.1039/d4mh00515e. Mater Horiz. 2024. PMID: 39139133 Review.
Cited by
-
A review on plasmonic-based heterojunction photocatalysts for degradation of organic pollutants in wastewater.J Mater Sci. 2023;58(15):6474-6515. doi: 10.1007/s10853-023-08391-w. Epub 2023 Mar 25. J Mater Sci. 2023. PMID: 37065680 Free PMC article. Review.
-
Enhanced coupling of perovskites with semiconductive properties by tuning multi-modal optically active nanostructured set-ups for photonics, photovoltaics and energy applications.RSC Adv. 2025 Feb 25;15(7):5571-5596. doi: 10.1039/d5ra00458f. eCollection 2025 Feb 13. RSC Adv. 2025. PMID: 40007863 Free PMC article. Review.
-
Ultra-Fast Charge Transfer in P3HT Composites Using the Core Hole Clock Technique.Nanomaterials (Basel). 2025 Mar 12;15(6):433. doi: 10.3390/nano15060433. Nanomaterials (Basel). 2025. PMID: 40137606 Free PMC article. Review.
-
Instantaneous Property Prediction and Inverse Design of Plasmonic Nanostructures Using Machine Learning: Current Applications and Future Directions.Nanomaterials (Basel). 2022 Feb 14;12(4):633. doi: 10.3390/nano12040633. Nanomaterials (Basel). 2022. PMID: 35214962 Free PMC article. Review.
-
The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study.Nanomaterials (Basel). 2022 Sep 10;12(18):3137. doi: 10.3390/nano12183137. Nanomaterials (Basel). 2022. PMID: 36144925 Free PMC article.
References
-
- BP . BP Statistical Review of World Energy. BP; London, UK: 2018.
-
- International Energy Agency . Key World Energy Statistics. International Energy Agency; Paris, France: 2018.
-
- U.S. Energy Information Administration . International Energy Outlook. U.S. Energy Information Administration; Washington, DC, USA: 2010.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

