Candida Administration in Bilateral Nephrectomy Mice Elevates Serum (1→3)-β-D-glucan That Enhances Systemic Inflammation Through Energy Augmentation in Macrophages
- PMID: 34068595
- PMCID: PMC8126065
- DOI: 10.3390/ijms22095031
Candida Administration in Bilateral Nephrectomy Mice Elevates Serum (1→3)-β-D-glucan That Enhances Systemic Inflammation Through Energy Augmentation in Macrophages
Abstract
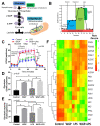
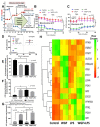
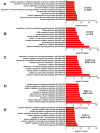
Systemic inflammation, from gut translocation of organismal molecules, might worsen uremic complications in acute kidney injury (AKI). The monitoring of gut permeability integrity and/or organismal molecules in AKI might be clinically beneficial. Due to the less prominence of Candida albicans in human intestine compared with mouse gut, C. albicans were orally administered in bilateral nephrectomy (BiN) mice. Gut dysbiosis, using microbiome analysis, and gut permeability defect (gut leakage), which was determined by fluorescein isothiocyanate-dextran and intestinal tight-junction immunofluorescent staining, in mice with BiN-Candida was more severe than BiN without Candida. Additionally, profound gut leakage in BiN-Candida also resulted in gut translocation of lipopolysaccharide (LPS) and (1→3)-β-D-glucan (BG), the organismal components from gut contents, that induced more severe systemic inflammation than BiN without Candida. The co-presentation of LPS and BG in mouse serum enhanced inflammatory responses. As such, LPS with Whole Glucan Particle (WGP, a representative BG) induced more severe macrophage responses than LPS alone as determined by supernatant cytokines and gene expression of downstream signals (NFκB, Malt-1 and Syk). Meanwhile, WGP alone did not induced the responses. In parallel, WGP (with or without LPS), but not LPS alone, accelerated macrophage ATP production (extracellular flux analysis) through the upregulation of genes in mitochondria and glycolysis pathway (using RNA sequencing analysis), without the induction of cell activities. These data indicated a WGP pre-conditioning effect on cell energy augmentation. In conclusion, Candida in BiN mice accelerated gut translocation of BG that augmented cell energy status and enhanced pro-inflammatory macrophage responses. Hence, gut fungi and BG were associated with the enhanced systemic inflammation in acute uremia.
Keywords: (1→3)-β-D-glucan; Candida; bilateral nephrectomy; endotoxin; uremia mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Candida Administration Worsens Uremia-Induced Gut Leakage in Bilateral Nephrectomy Mice, an Impact of Gut Fungi and Organismal Molecules in Uremia.mSystems. 2021 Jan 12;6(1):e01187-20. doi: 10.1128/mSystems.01187-20. mSystems. 2021. PMID: 33436518 Free PMC article.
-
Candida Administration Worsens Cecal Ligation and Puncture-Induced Sepsis in Obese Mice Through Gut Dysbiosis Enhanced Systemic Inflammation, Impact of Pathogen-Associated Molecules From Gut Translocation and Saturated Fatty Acid.Front Immunol. 2020 Sep 25;11:561652. doi: 10.3389/fimmu.2020.561652. eCollection 2020. Front Immunol. 2020. PMID: 33101279 Free PMC article.
-
Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (1➔3)-β-D-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34.Int J Mol Sci. 2022 Feb 24;23(5):2511. doi: 10.3390/ijms23052511. Int J Mol Sci. 2022. PMID: 35269654 Free PMC article.
-
Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis.Dig Dis Sci. 2019 Sep;64(9):2416-2428. doi: 10.1007/s10620-019-05581-y. Epub 2019 Mar 13. Dig Dis Sci. 2019. PMID: 30863955 Review.
-
Circulating LPS and (1→3)-β-D-Glucan: A Folie à Deux Contributing to HIV-Associated Immune Activation.Front Immunol. 2019 Mar 18;10:465. doi: 10.3389/fimmu.2019.00465. eCollection 2019. Front Immunol. 2019. PMID: 30967860 Free PMC article. Review.
Cited by
-
Blood Bacteria-Free DNA in Septic Mice Enhances LPS-Induced Inflammation in Mice through Macrophage Response.Int J Mol Sci. 2022 Feb 8;23(3):1907. doi: 10.3390/ijms23031907. Int J Mol Sci. 2022. PMID: 35163830 Free PMC article.
-
Candida Administration in 5/6 Nephrectomized Mice Enhanced Fibrosis in Internal Organs: An Impact of Lipopolysaccharide and (1→3)-β-D-Glucan from Leaky Gut.Int J Mol Sci. 2022 Dec 15;23(24):15987. doi: 10.3390/ijms232415987. Int J Mol Sci. 2022. PMID: 36555628 Free PMC article.
-
Sepsis Encephalopathy Is Partly Mediated by miR370-3p-Induced Mitochondrial Injury but Attenuated by BAM15 in Cecal Ligation and Puncture Sepsis Male Mice.Int J Mol Sci. 2022 May 13;23(10):5445. doi: 10.3390/ijms23105445. Int J Mol Sci. 2022. PMID: 35628259 Free PMC article.
-
Enhanced Bacteremia in Dextran Sulfate-Induced Colitis in Splenectomy Mice Correlates with Gut Dysbiosis and LPS Tolerance.Int J Mol Sci. 2022 Jan 31;23(3):1676. doi: 10.3390/ijms23031676. Int J Mol Sci. 2022. PMID: 35163596 Free PMC article.
-
Enhancing Immunity and Modulating Vaginal Microflora Against Candidal Vaginitis Through Nanoemulsion Supplemented with Porphyra Oligosaccharide as an Intravaginal Vaccine Adjuvant.Int J Nanomedicine. 2023 Nov 6;18:6333-6346. doi: 10.2147/IJN.S431009. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37954454 Free PMC article.
References
-
- Leelahavanichkul A., Huang Y., Hu X., Zhou H., Tsuji T., Chen R., Kopp J.B., Schnermann J., Yuen P.S., Star R.A. Chronic kidney disease worsens sepsis and sepsis—induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 2011;80:1198–1211. doi: 10.1038/ki.2011.261. - DOI - PMC - PubMed
-
- Leelahavanichkul A., Somparn P., Issara-Amphorn J., Eiam-ong S., Avihingsanon Y., Hirankarn N., Srisawat N. Serum Neutrophil Gelatinase Associated Lipocalin (NGAL) Outperforms Serum Creatinine in Detecting Sepsis-Induced Acute Kidney Injury, Experiments on Bilateral Nephrectomy and Bilateral Ureter Obstruction Mouse Models. Shock. 2016;45:570–576. doi: 10.1097/SHK.0000000000000530. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

