The Quest to Quantify Selective and Synergistic Effects of Plasma for Cancer Treatment: Insights from Mathematical Modeling
- PMID: 34068601
- PMCID: PMC8126141
- DOI: 10.3390/ijms22095033
The Quest to Quantify Selective and Synergistic Effects of Plasma for Cancer Treatment: Insights from Mathematical Modeling
Abstract
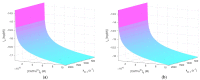
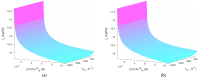
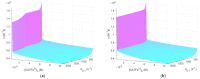
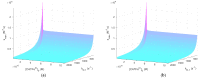
Cold atmospheric plasma (CAP) and plasma-treated liquids (PTLs) have recently become a promising option for cancer treatment, but the underlying mechanisms of the anti-cancer effect are still to a large extent unknown. Although hydrogen peroxide (H2O2) has been recognized as the major anti-cancer agent of PTL and may enable selectivity in a certain concentration regime, the co-existence of nitrite can create a synergistic effect. We develop a mathematical model to describe the key species and features of the cellular response toward PTL. From the numerical solutions, we define a number of dependent variables, which represent feasible measures to quantify cell susceptibility in terms of the H2O2 membrane diffusion rate constant and the intracellular catalase concentration. For each of these dependent variables, we investigate the regimes of selective versus non-selective, and of synergistic versus non-synergistic effect to evaluate their potential role as a measure of cell susceptibility. Our results suggest that the maximal intracellular H2O2 concentration, which in the selective regime is almost four times greater for the most susceptible cells compared to the most resistant cells, could be used to quantify the cell susceptibility toward exogenous H2O2. We believe our theoretical approach brings novelty to the field of plasma oncology, and more broadly, to the field of redox biology, by proposing new ways to quantify the selective and synergistic anti-cancer effect of PTL in terms of inherent cell features.
Keywords: cold atmospheric plasma; hydrogen peroxide; mathematical modeling; reaction network; selective cancer treatment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


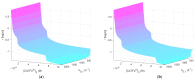


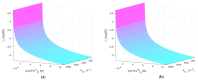
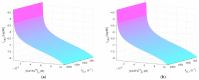
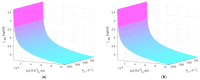





References
-
- Lin A., Truong B., Patel S., Kaushik N., Choi E.H., Fridman G., Fridman A., Miller V. Nanosecond-Pulsed DBD plasma-generated reactive oxygen species trigger immunogenic cell death in A549 lung carcinoma cells through intracellular oxidative stress. Int. J. Mol. Sci. 2017;18:966. doi: 10.3390/ijms18050966. - DOI - PMC - PubMed
-
- Lin A., Gorbanev Y., De Backer J., Van Loenhout J., Van Boxem W., Lemiere F., Cos P., Dewilde S., Smits E., Bogaerts A. Non-thermal plasma as a unique delivery system of short-lived reactive oxygen and nitrogen species for immunogenic cell death in melanoma cells. Adv. Sci. 2019;6:15. doi: 10.1002/advs.201802062. - DOI - PMC - PubMed
-
- Bisag A., Bucci C., Coluccelli S., Girolimetti G., Laurita R., De Iaco P., Perrone A.M., Gherardi M., Marchio L., Porcelli A.M., et al. Plasma-activated Ringer’s Lactate solution displays a selective cytotoxic effect on ovarian cancer cells. Cancers. 2020;12:476. doi: 10.3390/cancers12020476. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

