Biological Activity of Selected Natural and Synthetic Terpenoid Lactones
- PMID: 34068609
- PMCID: PMC8126056
- DOI: 10.3390/ijms22095036
Biological Activity of Selected Natural and Synthetic Terpenoid Lactones
Abstract
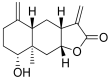
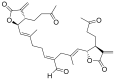
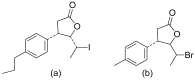
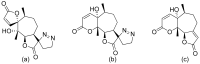
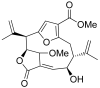
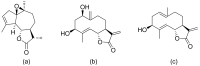
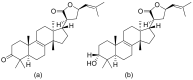
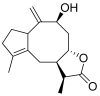
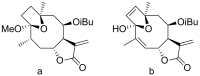
Terpenoids with lactone moieties have been indicated to possess high bioactivity. Certain terpenoid lactones exist in nature, in plants and animals, but they can also be obtained by chemical synthesis. Terpenoids possessing lactone moieties are known for their cytotoxic, anti-inflammatory, antimicrobial, anticancer, and antimalarial activities. Moreover, one terpenoid lactone, artemisinin, is used as a drug against malaria. Because of these abilities, there is constant interest in new terpenoid lactones that are both isolated and synthesized, and their biological activities have been verified. In some cases, the activity of the terpenoid lactone is specifically connected to the lactone moiety. Recent works have revealed that new terpenoid lactones can demonstrate such functions and are thus considered to be potential active agents against many diseases.
Keywords: biological activity; lactones; terpenoid.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





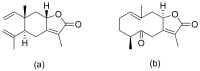



Similar articles
-
Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production.Planta Med. 2003 Apr;69(4):289-99. doi: 10.1055/s-2003-38871. Planta Med. 2003. PMID: 12709893 Review.
-
SANTAMARINE: Mechanistic studies on multiple diseases.Chem Biol Drug Des. 2020 Apr;95(4):427-434. doi: 10.1111/cbdd.13666. Epub 2020 Mar 5. Chem Biol Drug Des. 2020. PMID: 32022458 Review.
-
Antimalarial, antiproliferative, and antitumor activities of artemisinin-derived, chemically robust, trioxane dimers.J Med Chem. 1999 Oct 21;42(21):4275-80. doi: 10.1021/jm990363d. J Med Chem. 1999. PMID: 10543871
-
α-Methylene-γ-lactones as a novel class of anti-leukemic agents.Anticancer Agents Med Chem. 2014 Jun;14(5):688-94. doi: 10.2174/1871520614666140313095010. Anticancer Agents Med Chem. 2014. PMID: 24628266 Review.
-
Development of Anticancer Agents from Plant-Derived Sesquiterpene Lactones.Curr Med Chem. 2016;23(23):2397-420. doi: 10.2174/0929867323666160510123255. Curr Med Chem. 2016. PMID: 27160533 Free PMC article. Review.
Cited by
-
Antimicrobial Activity of Lactones.Antibiotics (Basel). 2022 Sep 29;11(10):1327. doi: 10.3390/antibiotics11101327. Antibiotics (Basel). 2022. PMID: 36289985 Free PMC article. Review.
-
Jasonia glutinosa (L.) DC.: Back in Our Pantries? A Review of Its Pharmacological Activity and Mechanisms of Action.Int J Mol Sci. 2025 Mar 12;26(6):2536. doi: 10.3390/ijms26062536. Int J Mol Sci. 2025. PMID: 40141177 Free PMC article. Review.
-
Hemistepsin A induces apoptosis by modulating the reactive oxygen species-dependent PI3K/Akt signaling pathway in human lung carcinoma A549 cells.Korean J Physiol Pharmacol. 2025 Sep 1;29(5):625-636. doi: 10.4196/kjpp.25.044. Epub 2025 Jul 24. Korean J Physiol Pharmacol. 2025. PMID: 40701842 Free PMC article.
-
Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds' Effects on a Glioblastoma Cellular Model.Pharmaceutics. 2023 Apr 30;15(5):1383. doi: 10.3390/pharmaceutics15051383. Pharmaceutics. 2023. PMID: 37242625 Free PMC article.
-
The Paradigm Shift of Using Natural Molecules Extracted from Northern Canada to Combat Malaria.Infect Dis Rep. 2024 Jun 26;16(4):543-560. doi: 10.3390/idr16040041. Infect Dis Rep. 2024. PMID: 39051241 Free PMC article. Review.
References
-
- Fittig R., Petri C. Untersuchungen über die ungesättigten Säuren. I. Weitere Beiträge zur Kenntnifs der Fumarsäure und Maleïnsäure. Justus Liebigs Ann. Chem. 1879;195:56–179. doi: 10.1002/jlac.18791950103. - DOI
-
- Buckle J. Clinical Aromatherapy. Elsevier; Amsterdam, The Netherlands: 2015. Basic Plant Taxonomy, Basic Essential Oil Chemistry, Extraction, Biosynthesis, and Analysis; pp. 37–72.
-
- Baser K.H., Demirci F. Chemistry of essential oils. In: Berger R.G., editor. Flavours and Fragrances-Chemistry, Bioprocessing and Sustainability. Springer; Berlin/Heidelberg, Germany: 2007. pp. 43–83.
-
- Siengalewicz P., Mulzer J., Rinner U. 6.09 Synthesis of Esters and Lactones. In: Knochel P., editor. Comprehensive Organic Synthesis. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2014. pp. 355–410.
-
- Mulzer J. 2.3 General Principles of Diastereoselective Reactions: Diastereoselectivity via Substrate-Directable Reactions (Internal Delivery) and Heterocyclizations. In: Carreira E.M., Yamamoto H., editors. Comprehensive Chirality. Elsevier; Amsterdam, The Netherlands: 2012. pp. 45–64.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

