N-[4-(N,N,N-Trimethylammonium)Benzyl]Chitosan Chloride as a Gene Carrier: The Influence of Polyplex Composition and Cell Type
- PMID: 34068680
- PMCID: PMC8126137
- DOI: 10.3390/ma14092467
N-[4-(N,N,N-Trimethylammonium)Benzyl]Chitosan Chloride as a Gene Carrier: The Influence of Polyplex Composition and Cell Type
Abstract
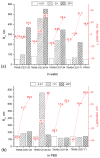
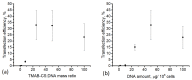
Polyplex-based gene delivery systems are promising substitutes for viral vectors because of their high versatility and lack of disadvantages commonly encountered with viruses. In this work, we studied the DNA polyplexes with N-[4-(N,N,N-trimethylammonium)benzyl]chitosan chloride (TMAB-CS) of various compositions in different cell types. Investigations of the interaction of TMAB-CS with DNA by different physical methods revealed that the molecular weight and the degree of substitution do not dramatically influence the hydrodynamic properties of polyplexes. Highly substituted TMAB-CS samples had a high affinity for DNA. The transfection protocol was optimized in HEK293T cells and achieved the highest efficiency of 30-35%. TMAB-CS was dramatically less effective in nonadherent K562 cells (around 1% transfected cells), but it was more effective and less toxic than polyarginine.
Keywords: cell transfection; chitosan; gene delivery; polyplex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Home—ClinicalTrials.Gov. [(accessed on 21 April 2021)]; Available online: https://clinicaltrials.gov/ct2/home.
Grants and funding
LinkOut - more resources
Full Text Sources

