OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs
- PMID: 34068720
- PMCID: PMC8151869
- DOI: 10.3390/cells10051148
OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs
Abstract
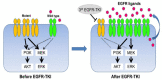
Background: Pharmacological targeting aberrant activation of epidermal growth factor receptor tyrosine kinase signaling is an established approach to treating lung adenocarcinoma. Osimertinib is a tyrosine kinase approved and effective in treating lung adenocarcinomas that have one of several common activating mutations in epidermal growth factor receptor. The emergence of resistance to osimertinib after a year or two is the rule. We developed a five-drug adjuvant regimen designed to increase osimertinib's growth inhibition and thereby delay the development of resistance. Areas of Uncertainty: Although the assembled preclinical data is strong, preclinical data and the following clinical trial results can be discrepant. The safety of OPALS drugs when used individually is excellent. We have no data from humans on their tolerability when used as an ensemble. That there is no data from the individual drugs to suspect problematic interaction does not exclude the possibility.
Data sources: All relevant PubMed.org articles on the OPALS drugs and corresponding pathophysiology of lung adenocarcinoma and glioblastoma were reviewed. Therapeutic Opinion: The five drugs of OPALS are in wide use in general medicine for non-oncology indications. OPALS uses the anti-protozoal drug pyrimethamine, the antihistamine cyproheptadine, the antibiotic azithromycin, the antihistamine loratadine, and the potassium sparing diuretic spironolactone. We show how these inexpensive and generically available drugs intersect with and inhibit lung adenocarcinoma growth drive. We also review data showing that both OPALS adjuvant drugs and osimertinib have data showing they may be active in suppressing glioblastoma growth.
Keywords: EGFR; NSCLC; cancer stem cells; glioblastoma; osimertinib; repurposing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Halatsch M.-E., Kast R.E., Dwucet A., Hlavac M., Heiland T., Westhoff M.-A., Debatin K.-M., Wirtz C.R., Siegelin M.D., Karpel-Massler G. Bcl-2/Bcl-xL inhibition predominantly synergistically enhances the anti-neoplastic activity of a low-dose CUSP9 repurposed drug regime against glioblastoma. Br. J. Pharmacol. 2019;176:3681–3694. doi: 10.1111/bph.14773. - DOI - PMC - PubMed
-
- Kast R.E., Karpel-Massler G., Halatsch M.-E. CUSP9* treatment protocol for recurrent glioblastoma: Aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget. 2014;5:8052–8082. doi: 10.18632/oncotarget.2408. - DOI - PMC - PubMed
-
- Kast R.E., Boockvar J.A., Brüning A., Cappello F., Chang W.-W., Cvek B., Dou Q.P., Duenas-Gonzalez A., Efferth T., Focosi D., et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget. 2013;4:502–530. doi: 10.18632/oncotarget.969. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous