CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus
- PMID: 34068752
- PMCID: PMC8151692
- DOI: 10.3390/cells10051151
CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus
Abstract
Background: To investigate the role of CD47 in inflammatory responses in systemic lupus erythematosus (SLE).
Methods: Expression of CD47 and signal regulatory protein alpha (SIRPα) by peripheral blood mononuclear cells (PBMCs) and changes in CD47 expression after exposure to SLE serum, healthy control (HC) serum, recombinant interferon (IFN)-α, or tumor necrosis factor (TNF)-α were examined. Human monocytes and THP1 cells were incubated with lipopolysaccharide (LPS), an anti-CD47 antibody, or both. TNF-α production was examined. Sera from SLE patients and HCs were screened to detect autoantibodies specific for CD47.
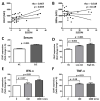
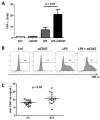
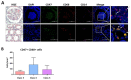
Results: Twenty-five SLE patients and sixteen HCs were enrolled. CD47 expression by monocytes from SLE patients was higher than those from HCs (mean fluorescence intensity ± SD: 815.9 ± 269.4 vs. 511.5 ± 199.4, respectively; p < 0.001). CD47 expression by monocytes correlated with SLE disease activity (Spearman's rho = 0.467, p = 0.019). IFN-α but not TNF-α, increased CD47 expression. Exposing monocytes to an anti-CD47 antibody plus LPS increased TNF-α production by 21.0 ± 10.9-fold (compared with 7.3 ± 5.5-fold for LPS alone). Finally, levels of autoantibodies against CD47 were higher in SLE patients than in HCs (21.4 ± 7.1 ng/mL vs. 16.1 ± 3.1 ng/mL, respectively; p = 0.02). Anti-CD47 antibody levels did not correlate with disease activity (Spearman's rho = -0.11, p = 0.759) or CD47 expression on CD14 monocytes (Spearman's rho = 0.079, p = 0.838) in patients.
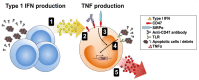
Conclusions: CD47 expression by monocytes is upregulated in SLE and correlates with disease activity. CD47 contributes to augmented inflammatory responses in SLE. Targeting CD47 might be a novel treatment for SLE.
Keywords: CD47; SIRP-alpha; inflammatory response; systemic lupus erythematosus.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

