Oxidase Reactivity of CuII Bound to N-Truncated Aβ Peptides Promoted by Dopamine
- PMID: 34068879
- PMCID: PMC8155989
- DOI: 10.3390/ijms22105190
Oxidase Reactivity of CuII Bound to N-Truncated Aβ Peptides Promoted by Dopamine
Abstract
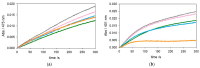
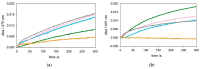
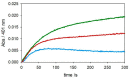
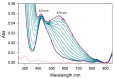
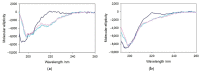
The redox chemistry of copper(II) is strongly modulated by the coordination to amyloid-β peptides and by the stability of the resulting complexes. Amino-terminal copper and nickel binding motifs (ATCUN) identified in truncated Aβ sequences starting with Phe4 show very high affinity for copper(II) ions. Herein, we study the oxidase activity of [Cu-Aβ4-x] and [Cu-Aβ1-x] complexes toward dopamine and other catechols. The results show that the CuII-ATCUN site is not redox-inert; the reduction of the metal is induced by coordination of catechol to the metal and occurs through an inner sphere reaction. The generation of a ternary [CuII-Aβ-catechol] species determines the efficiency of the oxidation, although the reaction rate is ruled by reoxidation of the CuI complex. In addition to the N-terminal coordination site, the two vicinal histidines, His13 and His14, provide a second Cu-binding motif. Catechol oxidation studies together with structural insight from the mixed dinuclear complexes Ni/Cu-Aβ4-x reveal that the His-tandem is able to bind CuII ions independently of the ATCUN site, but the N-terminal metal complexation reduces the conformational mobility of the peptide chain, preventing the binding and oxidative reactivity toward catechol of CuII bound to the secondary site.
Keywords: Alzheimer’s disease; amyloid-β peptides; copper; dopamine; neurodegeneration; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Using N-Terminal Coordination of Cu(II) and Ni(II) to Isolate the Coordination Environment of Cu(I) and Cu(II) Bound to His13 and His14 in Amyloid-β(4-16).Inorg Chem. 2019 Nov 18;58(22):15138-15154. doi: 10.1021/acs.inorgchem.9b01940. Epub 2019 Oct 28. Inorg Chem. 2019. PMID: 31657204
-
Structural Insight into Redox Dynamics of Copper Bound N-Truncated Amyloid-β Peptides from in Situ X-ray Absorption Spectroscopy.Inorg Chem. 2018 Sep 17;57(18):11422-11435. doi: 10.1021/acs.inorgchem.8b01255. Epub 2018 Aug 31. Inorg Chem. 2018. PMID: 30169035
-
The Palladium(II) Complex of Aβ4-16 as Suitable Model for Structural Studies of Biorelevant Copper(II) Complexes of N-Truncated Beta-Amyloids.Int J Mol Sci. 2020 Dec 2;21(23):9200. doi: 10.3390/ijms21239200. Int J Mol Sci. 2020. PMID: 33276669 Free PMC article.
-
CuII Binding Properties of N-Truncated Aβ Peptides: In Search of Biological Function.Inorg Chem. 2019 Oct 21;58(20):13561-13577. doi: 10.1021/acs.inorgchem.9b01399. Epub 2019 Jul 15. Inorg Chem. 2019. PMID: 31304745 Review.
-
Kinetics of Cu(II) complexation by ATCUN/NTS and related peptides: a gold mine of novel ideas for copper biology.Dalton Trans. 2021 Dec 20;51(1):14-26. doi: 10.1039/d1dt02878b. Dalton Trans. 2021. PMID: 34816848 Review.
Cited by
-
A Focus on the Link Between Metal Dyshomeostasis, Norepinephrine, and Protein Aggregation.Antioxidants (Basel). 2025 Mar 15;14(3):347. doi: 10.3390/antiox14030347. Antioxidants (Basel). 2025. PMID: 40227404 Free PMC article. Review.
-
Peptides Derived from Angiogenin Regulate Cellular Copper Uptake.Int J Mol Sci. 2021 Sep 2;22(17):9530. doi: 10.3390/ijms22179530. Int J Mol Sci. 2021. PMID: 34502439 Free PMC article.
References
-
- Portelius E., Bogdanovic N., Gustavsson M.K., Volkmann I., Brinkmalm G., Zetterberg H., Winblad B., Blennow K. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2010;120:185–193. doi: 10.1007/s00401-010-0690-1. - DOI - PMC - PubMed
-
- Bouter Y., Dietrich K., Wittnam J.L., Rezaei-Ghaleh N., Pillot T., Papot-Couturier S., Lefebvre T., Sprenger F., Wirths O., Zweckstetter M., et al. N-truncated amyloid β (Aβ) 4-42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol. 2013;126:189–205. doi: 10.1007/s00401-013-1129-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

