Oxidase Reactivity of CuII Bound to N-Truncated Aβ Peptides Promoted by Dopamine
- PMID: 34068879
- PMCID: PMC8155989
- DOI: 10.3390/ijms22105190
Oxidase Reactivity of CuII Bound to N-Truncated Aβ Peptides Promoted by Dopamine
Abstract
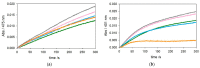
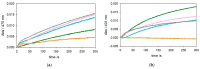
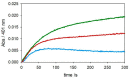
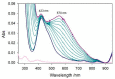
The redox chemistry of copper(II) is strongly modulated by the coordination to amyloid-β peptides and by the stability of the resulting complexes. Amino-terminal copper and nickel binding motifs (ATCUN) identified in truncated Aβ sequences starting with Phe4 show very high affinity for copper(II) ions. Herein, we study the oxidase activity of [Cu-Aβ4-x] and [Cu-Aβ1-x] complexes toward dopamine and other catechols. The results show that the CuII-ATCUN site is not redox-inert; the reduction of the metal is induced by coordination of catechol to the metal and occurs through an inner sphere reaction. The generation of a ternary [CuII-Aβ-catechol] species determines the efficiency of the oxidation, although the reaction rate is ruled by reoxidation of the CuI complex. In addition to the N-terminal coordination site, the two vicinal histidines, His13 and His14, provide a second Cu-binding motif. Catechol oxidation studies together with structural insight from the mixed dinuclear complexes Ni/Cu-Aβ4-x reveal that the His-tandem is able to bind CuII ions independently of the ATCUN site, but the N-terminal metal complexation reduces the conformational mobility of the peptide chain, preventing the binding and oxidative reactivity toward catechol of CuII bound to the secondary site.
Keywords: Alzheimer’s disease; amyloid-β peptides; copper; dopamine; neurodegeneration; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Portelius E., Bogdanovic N., Gustavsson M.K., Volkmann I., Brinkmalm G., Zetterberg H., Winblad B., Blennow K. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2010;120:185–193. doi: 10.1007/s00401-010-0690-1. - DOI - PMC - PubMed
-
- Bouter Y., Dietrich K., Wittnam J.L., Rezaei-Ghaleh N., Pillot T., Papot-Couturier S., Lefebvre T., Sprenger F., Wirths O., Zweckstetter M., et al. N-truncated amyloid β (Aβ) 4-42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol. 2013;126:189–205. doi: 10.1007/s00401-013-1129-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

