Theoretical Study on the Photo-Oxidation and Photoreduction of an Azetidine Derivative as a Model of DNA Repair
- PMID: 34068908
- PMCID: PMC8157190
- DOI: 10.3390/molecules26102911
Theoretical Study on the Photo-Oxidation and Photoreduction of an Azetidine Derivative as a Model of DNA Repair
Abstract
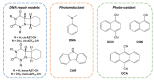
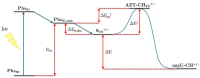
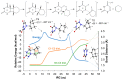
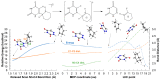
Photocycloreversion plays a central role in the study of the repair of DNA lesions, reverting them into the original pyrimidine nucleobases. Particularly, among the proposed mechanisms for the repair of DNA (6-4) photoproducts by photolyases, it has been suggested that it takes place through an intermediate characterized by a four-membered heterocyclic oxetane or azetidine ring, whose opening requires the reduction of the fused nucleobases. The specific role of this electron transfer step and its impact on the ring opening energetics remain to be understood. These processes are studied herein by means of quantum-chemical calculations on the two azetidine stereoisomers obtained from photocycloaddition between 6-azauracil and cyclohexene. First, we analyze the efficiency of the electron-transfer processes by computing the redox properties of the azetidine isomers as well as those of a series of aromatic photosensitizers acting as photoreductants and photo-oxidants. We find certain stereodifferentiation favoring oxidation of the cis-isomer, in agreement with previous experimental data. Second, we determine the reaction profiles of the ring-opening mechanism of the cationic, neutral, and anionic systems and assess their feasibility based on their energy barrier heights and the stability of the reactants and products. Results show that oxidation largely decreases the ring-opening energy barrier for both stereoisomers, even though the process is forecast as too slow to be competitive. Conversely, one-electron reduction dramatically facilitates the ring opening of the azetidine heterocycle. Considering the overall quantum-chemistry findings, N,N-dimethylaniline is proposed as an efficient photosensitizer to trigger the photoinduced cycloreversion of the DNA lesion model.
Keywords: DNA repair; azetidine; density functional theory; electron transfer; photochemistry; redox properties; ring opening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Experimental and Theoretical Study on the Cycloreversion of a Nucleobase-Derived Azetidine by Photoinduced Electron Transfer.Chemistry. 2018 Oct 12;24(57):15346-15354. doi: 10.1002/chem.201803298. Epub 2018 Sep 11. Chemistry. 2018. PMID: 30053323
-
Stereoselective Fluorescence Quenching in the Electron Transfer Photooxidation of Nucleobase-Related Azetidines by Cyanoaromatics.Molecules. 2016 Dec 7;21(12):1683. doi: 10.3390/molecules21121683. Molecules. 2016. PMID: 27941606 Free PMC article.
-
Electron-transfer induced repair of 6-4 photoproducts in DNA: a computational study.J Phys Chem A. 2007 Mar 29;111(12):2351-61. doi: 10.1021/jp0676383. Epub 2007 Mar 7. J Phys Chem A. 2007. PMID: 17388321
-
Insights into Light-driven DNA Repair by Photolyases: Challenges and Opportunities for Electronic Structure Theory.Photochem Photobiol. 2017 Jan;93(1):37-50. doi: 10.1111/php.12679. Epub 2017 Jan 5. Photochem Photobiol. 2017. PMID: 27925218 Review.
-
Biosyntheses of azetidine-containing natural products.Org Biomol Chem. 2023 Sep 20;21(36):7242-7254. doi: 10.1039/d3ob01205k. Org Biomol Chem. 2023. PMID: 37642579 Review.
Cited by
-
Reductive Photocycloreversion of Cyclobutane Dimers Triggered by Guanines.J Org Chem. 2023 Jul 21;88(14):10111-10121. doi: 10.1021/acs.joc.3c00930. Epub 2023 Jul 12. J Org Chem. 2023. PMID: 37437138 Free PMC article.
-
Spatial and Temporal Resolution of the Oxygen-Independent Photoinduced DNA Interstrand Cross-Linking by a Nitroimidazole Derivative.J Chem Inf Model. 2022 Jul 11;62(13):3239-3252. doi: 10.1021/acs.jcim.2c00460. Epub 2022 Jun 30. J Chem Inf Model. 2022. PMID: 35771238 Free PMC article.
References
-
- Francés-Monerris A., Gillet N., Dumont E., Monari A. QM/MM Studies of Light-Responsive Biological Systems. Springer; Cham, Switzerland: 2021. DNA photodamage and repair: Computational photobiology in action; pp. 293–332.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

