Autonomic Nervous System in Obesity and Insulin-Resistance-The Complex Interplay between Leptin and Central Nervous System
- PMID: 34068919
- PMCID: PMC8156658
- DOI: 10.3390/ijms22105187
Autonomic Nervous System in Obesity and Insulin-Resistance-The Complex Interplay between Leptin and Central Nervous System
Abstract
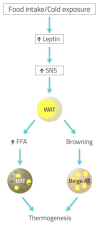
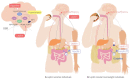
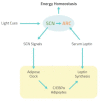
The role of the autonomic nervous system in obesity and insulin-resistant conditions has been largely explored. However, the exact mechanisms involved in this relation have not been completely elucidated yet, since most of these mechanisms display a bi-directional effect. Insulin-resistance, for instance, can be caused by sympathetic activation, but, in turn, the associated hyperinsulinemia can activate the sympathetic branch of the autonomic nervous system. The picture is made even more complex by the implicated neural, hormonal and nutritional mechanisms. Among them, leptin plays a pivotal role, being involved not only in appetite regulation and glucose homeostasis but also in energy expenditure. The purpose of this review is to offer a comprehensive view of the complex interplay between leptin and the central nervous system, providing further insights on the impact of autonomic nervous system balance on adipose tissue and insulin-resistance. Furthermore, the link between the circadian clock and leptin and its effect on metabolism and energy balance will be evaluated.
Keywords: autonomic nervous system (ANS); central nervous system (CNS); insulin-resistance; leptin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

