Regulation of DNA Replication Licensing and Re-Replication by Cdt1
- PMID: 34068957
- PMCID: PMC8155957
- DOI: 10.3390/ijms22105195
Regulation of DNA Replication Licensing and Re-Replication by Cdt1
Abstract
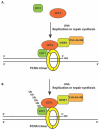
In eukaryotic cells, DNA replication licensing is precisely regulated to ensure that the initiation of genomic DNA replication in S phase occurs once and only once for each mitotic cell division. A key regulatory mechanism by which DNA re-replication is suppressed is the S phase-dependent proteolysis of Cdt1, an essential replication protein for licensing DNA replication origins by loading the Mcm2-7 replication helicase for DNA duplication in S phase. Cdt1 degradation is mediated by CRL4Cdt2 ubiquitin E3 ligase, which further requires Cdt1 binding to proliferating cell nuclear antigen (PCNA) through a PIP box domain in Cdt1 during DNA synthesis. Recent studies found that Cdt2, the specific subunit of CRL4Cdt2 ubiquitin E3 ligase that targets Cdt1 for degradation, also contains an evolutionarily conserved PIP box-like domain that mediates the interaction with PCNA. These findings suggest that the initiation and elongation of DNA replication or DNA damage-induced repair synthesis provide a novel mechanism by which Cdt1 and CRL4Cdt2 are both recruited onto the trimeric PCNA clamp encircling the replicating DNA strands to promote the interaction between Cdt1 and CRL4Cdt2. The proximity of PCNA-bound Cdt1 to CRL4Cdt2 facilitates the destruction of Cdt1 in response to DNA damage or after DNA replication initiation to prevent DNA re-replication in the cell cycle. CRL4Cdt2 ubiquitin E3 ligase may also regulate the degradation of other PIP box-containing proteins, such as CDK inhibitor p21 and histone methylase Set8, to regulate DNA replication licensing, cell cycle progression, DNA repair, and genome stability by directly interacting with PCNA during DNA replication and repair synthesis.
Keywords: CRL4Cdt2; Cdt1; Cdt2; DNA re-replication; DNA repair synthesis; DNA replication; PCNA; Replication licensing; genome instability.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Proliferating cell nuclear antigen interacts with the CRL4 ubiquitin ligase subunit CDT2 in DNA synthesis-induced degradation of CDT1.J Biol Chem. 2018 Dec 7;293(49):18879-18889. doi: 10.1074/jbc.RA118.003049. Epub 2018 Oct 9. J Biol Chem. 2018. PMID: 30301766 Free PMC article.
-
CDK1-dependent inhibition of the E3 ubiquitin ligase CRL4CDT2 ensures robust transition from S Phase to Mitosis.J Biol Chem. 2015 Jan 2;290(1):556-67. doi: 10.1074/jbc.M114.614701. Epub 2014 Nov 19. J Biol Chem. 2015. PMID: 25411249 Free PMC article.
-
Selective ubiquitylation of p21 and Cdt1 by UBCH8 and UBE2G ubiquitin-conjugating enzymes via the CRL4Cdt2 ubiquitin ligase complex.Mol Cell Biol. 2011 Aug;31(15):3136-45. doi: 10.1128/MCB.05496-11. Epub 2011 May 31. Mol Cell Biol. 2011. PMID: 21628527 Free PMC article.
-
CRL4Cdt2 Ubiquitin Ligase, A Genome Caretaker Controlled by Cdt2 Binding to PCNA and DNA.Genes (Basel). 2022 Jan 29;13(2):266. doi: 10.3390/genes13020266. Genes (Basel). 2022. PMID: 35205311 Free PMC article. Review.
-
The Licensing Factor Cdt1 Links Cell Cycle Progression to the DNA Damage Response.Anticancer Res. 2020 May;40(5):2449-2456. doi: 10.21873/anticanres.14214. Anticancer Res. 2020. PMID: 32366388 Review.
Cited by
-
CDT1 is a Potential Therapeutic Target for the Progression of NAFLD to HCC and the Exacerbation of Cancer.Curr Genomics. 2025;26(3):225-243. doi: 10.2174/0113892029313473240919105819. Epub 2024 Oct 4. Curr Genomics. 2025. PMID: 40433415 Free PMC article.
-
Precision Oncology with Drugs Targeting the Replication Stress, ATR, and Schlafen 11.Cancers (Basel). 2021 Sep 14;13(18):4601. doi: 10.3390/cancers13184601. Cancers (Basel). 2021. PMID: 34572827 Free PMC article. Review.
-
Coprophagy Prevention Decreases the Reproductive Performance and Granulosa Cell Apoptosis via Regulation of CTSB Gene in Rabbits.Front Physiol. 2022 Jul 18;13:926795. doi: 10.3389/fphys.2022.926795. eCollection 2022. Front Physiol. 2022. PMID: 35923240 Free PMC article.
-
HN1 Is Enriched in the S-Phase, Phosphorylated in Mitosis, and Contributes to Cyclin B1 Degradation in Prostate Cancer Cells.Biology (Basel). 2023 Jan 26;12(2):189. doi: 10.3390/biology12020189. Biology (Basel). 2023. PMID: 36829467 Free PMC article.
-
DNA replication: Mechanisms and therapeutic interventions for diseases.MedComm (2020). 2023 Feb 5;4(1):e210. doi: 10.1002/mco2.210. eCollection 2023 Feb. MedComm (2020). 2023. PMID: 36776764 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

