The TRPA1 Agonist Cinnamaldehyde Induces the Secretion of HCO3- by the Porcine Colon
- PMID: 34068986
- PMCID: PMC8156935
- DOI: 10.3390/ijms22105198
The TRPA1 Agonist Cinnamaldehyde Induces the Secretion of HCO3- by the Porcine Colon
Abstract
A therapeutic potential of the TRPA1 channel agonist cinnamaldehyde for use in inflammatory bowel disease is emerging, but the mechanisms are unclear. Semi-quantitative qPCR of various parts of the porcine gastrointestinal tract showed that mRNA for TRPA1 was highest in the colonic mucosa. In Ussing chambers, 1 mmol·L-1 cinnamaldehyde induced increases in short circuit current (ΔIsc) and conductance (ΔGt) across the colon that were higher than those across the jejunum or after 1 mmol·L-1 thymol. Lidocaine, amiloride or bumetanide did not change the response. The application of 1 mmol·L-1 quinidine or the bilateral replacement of 120 Na+, 120 Cl- or 25 HCO3- reduced ΔGt, while the removal of Ca2+ enhanced ΔGt with ΔIsc numerically higher. ΔIsc decreased after 0.5 NPPB, 0.01 indometacin and the bilateral replacement of 120 Na+ or 25 HCO3-. The removal of 120 Cl- had no effect. Cinnamaldehyde also activates TRPV3, but comparative measurements involving patch clamp experiments on overexpressing cells demonstrated that much higher concentrations are required. We suggest that cinnamaldehyde stimulates the secretion of HCO3- via apical CFTR and basolateral Na+-HCO3- cotransport, preventing acidosis and damage to the epithelium and the colonic microbiome. Signaling may involve the opening of TRPA1, depolarization of the epithelium and a rise in PGE2 following a lower uptake of prostaglandins via OATP2A1.
Keywords: TRPA1; TRPV3; Ussing chamber; cinnamaldehyde; colon; colonic buffering; epithelial transport; essential oils; intestine; patch clamp; pig; prostaglandin.
Conflict of interest statement
D.M., G.M. and F.S. declare no conflict of interests. At the time of the study, H.-S.B. and J.R. were employees of PerformaNat GmbH. There was no influence on the results during the evaluation and interpretation.
Figures



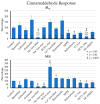



 ). The selectivity filter of the channel allows both the influx of Na+ and Ca2+, and a smaller efflux of K+, so that the effects on Isc are small. However, the cell is depolarised and a significant increase in conductance ΔGt is observed, which is reduced by quinidine and enhanced by the removal of divalent cations. The effects of cinnamaldehyde on TRPV3 (➀), which favors efflux of K+ over influx of Na+ and Ca2+, are discrete. Thymol opens both channels. (b) Prostanoids such as PGE2 are anions that are synthesized from membrane phospholipids via cyclooxygenase-mediated pathways and secreted into the extracellular space via pathways that are being explored (➁). For prostanoid signalling to end, the anionic prostaglandin has to be taken up into the cytosol via an electrogenic anion exchanger, OATP2A1 (SLCOA1) (➂), after which the prostaglandin is degraded by cytosolic enzymes. Due to the electrogenic nature of the cotransporter, the depolarization of the cellular membrane, as occurs after the opening of TRPA1 channels via cinnamaldehyde (
). The selectivity filter of the channel allows both the influx of Na+ and Ca2+, and a smaller efflux of K+, so that the effects on Isc are small. However, the cell is depolarised and a significant increase in conductance ΔGt is observed, which is reduced by quinidine and enhanced by the removal of divalent cations. The effects of cinnamaldehyde on TRPV3 (➀), which favors efflux of K+ over influx of Na+ and Ca2+, are discrete. Thymol opens both channels. (b) Prostanoids such as PGE2 are anions that are synthesized from membrane phospholipids via cyclooxygenase-mediated pathways and secreted into the extracellular space via pathways that are being explored (➁). For prostanoid signalling to end, the anionic prostaglandin has to be taken up into the cytosol via an electrogenic anion exchanger, OATP2A1 (SLCOA1) (➂), after which the prostaglandin is degraded by cytosolic enzymes. Due to the electrogenic nature of the cotransporter, the depolarization of the cellular membrane, as occurs after the opening of TRPA1 channels via cinnamaldehyde ( ), decreases the uptake of prostaglandins and thus increases the extracellular prostaglandin concentration. (c) After the binding of the PGE2 to EP4 receptors (➃) expressed by the colonic mucosa, adenylyl cyclase is stimulated, resulting in rising levels of cAMP that open apical CFTR channels (➄). Other anion channels may contribute to the secretion of HCO3−, which is driven by the uptake of Na+ via basolateral NBCn1 (Slc4a7), NBCe1 (SLC4A4), or NBCe2 (Slc4a5) at a ratio of 1, 2 or 3 HCO3− for each Na+ (➅). Most of the NPPB-sensitive rise in Isc that is observed after the activation of TRPA1 via cinnamaldehyde can be explained by this mechanism. The secretion of HCO3− is important for the buffering of protons formed in the fermentational process (➆), and for the unfolding of mucines in the mucus layer, thus protecting the epithelium. Energy-rich short chain fatty acid anions (SCFA−) are absorbed via various transport proteins (➇) without challenging cytosolic pH homeostasis. In physiological concentrations, prostaglandins are also thought to have barrier-enhancing properties through interaction with tight junction proteins (➈). Possibly, the secretion of HCO3− is highest in cells near the surface, while in the crypts, the expression of NKCC1 (SLC12A2) (➉) predominates. The latter pathway leads to the secretion of Cl− via CFTR, which can result in diarrhea when cAMP levels are pathologically high. Because the gradients favor a unilateral efflux of anions, the opening of CFTR will have higher effects on ΔIsc than those after the opening of TRPA1.
), decreases the uptake of prostaglandins and thus increases the extracellular prostaglandin concentration. (c) After the binding of the PGE2 to EP4 receptors (➃) expressed by the colonic mucosa, adenylyl cyclase is stimulated, resulting in rising levels of cAMP that open apical CFTR channels (➄). Other anion channels may contribute to the secretion of HCO3−, which is driven by the uptake of Na+ via basolateral NBCn1 (Slc4a7), NBCe1 (SLC4A4), or NBCe2 (Slc4a5) at a ratio of 1, 2 or 3 HCO3− for each Na+ (➅). Most of the NPPB-sensitive rise in Isc that is observed after the activation of TRPA1 via cinnamaldehyde can be explained by this mechanism. The secretion of HCO3− is important for the buffering of protons formed in the fermentational process (➆), and for the unfolding of mucines in the mucus layer, thus protecting the epithelium. Energy-rich short chain fatty acid anions (SCFA−) are absorbed via various transport proteins (➇) without challenging cytosolic pH homeostasis. In physiological concentrations, prostaglandins are also thought to have barrier-enhancing properties through interaction with tight junction proteins (➈). Possibly, the secretion of HCO3− is highest in cells near the surface, while in the crypts, the expression of NKCC1 (SLC12A2) (➉) predominates. The latter pathway leads to the secretion of Cl− via CFTR, which can result in diarrhea when cAMP levels are pathologically high. Because the gradients favor a unilateral efflux of anions, the opening of CFTR will have higher effects on ΔIsc than those after the opening of TRPA1.References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

