Optimization of the Biosynthesis of B-Ring Ortho-Hydroxy Lated Flavonoids Using the 4-Hydroxyphenylacetate 3-Hydroxylase Complex (HpaBC) of Escherichia coli
- PMID: 34069009
- PMCID: PMC8156182
- DOI: 10.3390/molecules26102919
Optimization of the Biosynthesis of B-Ring Ortho-Hydroxy Lated Flavonoids Using the 4-Hydroxyphenylacetate 3-Hydroxylase Complex (HpaBC) of Escherichia coli
Abstract
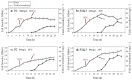
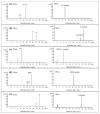
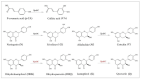
Flavonoids are important plant metabolites that exhibit a wide range of physiological and pharmaceutical functions. Because of their wide biological activities, such as anti-inflammatory, antioxidant, antiaging and anticancer, they have been widely used in foods, nutraceutical and pharmaceuticals industries. Here, the hydroxylase complex HpaBC was selected for the efficient in vivo production of ortho-hydroxylated flavonoids. Several HpaBC expression vectors were constructed, and the corresponding products were successfully detected by feeding naringenin to vector-carrying strains. However, when HpaC was linked with an S-Tag on the C terminus, the enzyme activity was significantly affected. The optimal culture conditions were determined, including a substrate concentration of 80 mg·L-1, an induction temperature of 28 °C, an M9 medium, and a substrate delay time of 6 h after IPTG induction. Finally, the efficiency of eriodictyol conversion from P2&3-carrying strains fed naringin was up to 57.67 ± 3.36%. The same strategy was used to produce catechin and caffeic acid, and the highest conversion efficiencies were 35.2 ± 3.14 and 32.93 ± 2.01%, respectively. In this paper, the catalytic activity of HpaBC on dihydrokaempferol and kaempferol was demonstrated for the first time. This study demonstrates a feasible method for efficiently synthesizing in vivo B-ring dihydroxylated flavonoids, such as catechins, flavanols, dihydroflavonols and flavonols, in a bacterial expression system.
Keywords: 4-hydroxyphenylacetate 3-hydroxylase; B-ring ortho-hydroxylation; Escherichia coli; biosynthesis; flavonoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Advances in 4-Hydroxyphenylacetate-3-hydroxylase Monooxygenase.Molecules. 2023 Sep 19;28(18):6699. doi: 10.3390/molecules28186699. Molecules. 2023. PMID: 37764475 Free PMC article. Review.
-
Optimization of naringenin and p-coumaric acid hydroxylation using the native E. coli hydroxylase complex, HpaBC.Biotechnol Prog. 2016 Jan-Feb;32(1):21-5. doi: 10.1002/btpr.2185. Epub 2015 Nov 11. Biotechnol Prog. 2016. PMID: 26488898
-
Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex.Microb Cell Fact. 2012 Apr 4;11:42. doi: 10.1186/1475-2859-11-42. Microb Cell Fact. 2012. PMID: 22475509 Free PMC article.
-
Production of bioactive hydroxyflavones by using monooxygenase from Saccharothrix espanaensis.J Biotechnol. 2014 Apr 20;176:11-7. doi: 10.1016/j.jbiotec.2014.02.002. Epub 2014 Feb 19. J Biotechnol. 2014. PMID: 24560623
-
The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis.Int J Mol Sci. 2014 Jan 15;15(1):1080-95. doi: 10.3390/ijms15011080. Int J Mol Sci. 2014. PMID: 24434621 Free PMC article. Review.
Cited by
-
Efficient production of hydroxysalidroside in Escherichia coli via enhanced glycosylation and semi-rational design of UGT85A1.Synth Syst Biotechnol. 2025 Mar 6;10(2):638-649. doi: 10.1016/j.synbio.2025.03.002. eCollection 2025 Jun. Synth Syst Biotechnol. 2025. PMID: 40166613 Free PMC article.
-
Advances in 4-Hydroxyphenylacetate-3-hydroxylase Monooxygenase.Molecules. 2023 Sep 19;28(18):6699. doi: 10.3390/molecules28186699. Molecules. 2023. PMID: 37764475 Free PMC article. Review.
-
4-Hydroxyphenylacetate 3-Hydroxylase (4HPA3H): A Vigorous Monooxygenase for Versatile O-Hydroxylation Applications in the Biosynthesis of Phenolic Derivatives.Int J Mol Sci. 2024 Jan 19;25(2):1222. doi: 10.3390/ijms25021222. Int J Mol Sci. 2024. PMID: 38279222 Free PMC article. Review.
-
Improvement of the Quality of Wild Rocket (Diplotaxis tenuifolia) with Respect to Health-Related Compounds by Enhanced Growth Irradiance.J Agric Food Chem. 2024 May 1;72(17):9735-9745. doi: 10.1021/acs.jafc.3c07698. Epub 2024 Apr 22. J Agric Food Chem. 2024. PMID: 38648561 Free PMC article.
-
Biosynthesis of eriodictyol from tyrosine by Corynebacterium glutamicum.Microb Cell Fact. 2022 May 14;21(1):86. doi: 10.1186/s12934-022-01815-3. Microb Cell Fact. 2022. PMID: 35568867 Free PMC article.
References
-
- Nair S., Gupta R. Dietary antioxidant flavonoids and coronary heart disease. J. Assoc. Phys. India. 1996;44:699–702. - PubMed
-
- Mishra P.K., Raghuram G.V., Bhargava A., Ahirwar A., Samarth R., Upadhyaya R., Jain S.K., Pathak N. In vitro and in vivo evaluation of the anticarcinogenic and cancer chemopreventive potential of a flavonoid-rich fraction from a traditional Indian herb Selaginella bryopteris. Br. J. Nutr. 2011;106:1154–1168. doi: 10.1017/S0007114511001498. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

