The Novel Zoonotic Pathogen, Anaplasma capra, Infects Human Erythrocytes, HL-60, and TF-1 Cells In Vitro
- PMID: 34069112
- PMCID: PMC8156996
- DOI: 10.3390/pathogens10050600
The Novel Zoonotic Pathogen, Anaplasma capra, Infects Human Erythrocytes, HL-60, and TF-1 Cells In Vitro
Abstract
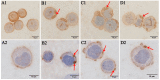
Anaplasma capra, a species of the family Anaplasmataceae, is zoonotic tick-borne obligate intracellular bacteria. There have been no reports of human infection with this pathogen since 2015. Therefore, the zoonotic characteristics of A. capra need to be further studied. To verify the ability of A. capra to infect human cells, A. capra were inoculated in human erythrocytes, HL-60, and TF-1 cell lines in vitro. Cell smears were taken after inoculation, using Giemsa staining, transmission electron microscope (TEM), chromogenic in situ hybridization and immunocytochemistry for detection. In the Giemsa staining, many dark colored corpuscles or purple granules were seen in the inoculated erythrocytes, HL-60, and TF-1 cells. The results of chromogenic in situ hybridization show that there were brown precipitates on the surface of most erythrocytes. Immunocytochemistry results show many dark brown vacuolar structures or corpuscles in the cytoplasm of erythrocytes, HL-60, and TF-1 cell lines. The A. capra morulae were seen in the cytoplasm of both HL-60 and TF-1 in TEM, and their diameter was about 295-518 nm. Both dense-cored (DC) and reticulate cell (RC) form morulae could be seen. This study confirmed the ability of A. capra to infect human erythrocytes, HL-60, and TF-1. This study is of profound significance in further verifying the zoonotic characteristics of the pathogen and for establishing an in vitro cultivation model.
Keywords: Anaplasma capra; HL-60; TF-1; erythrocyte; zoonotic pathogen.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



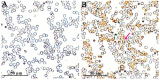

Similar articles
-
The first detection of Anaplasma capra, an emerging zoonotic Anaplasma sp., in erythrocytes.Emerg Microbes Infect. 2021 Dec;10(1):226-234. doi: 10.1080/22221751.2021.1876532. Emerg Microbes Infect. 2021. PMID: 33446064 Free PMC article.
-
Dogs as New Hosts for the Emerging Zoonotic Pathogen Anaplasma capra in China.Front Cell Infect Microbiol. 2019 Nov 26;9:394. doi: 10.3389/fcimb.2019.00394. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31850236 Free PMC article.
-
Differential identification of Anaplasma in cattle and potential of cattle to serve as reservoirs of Anaplasma capra, an emerging tick-borne zoonotic pathogen.Vet Microbiol. 2018 Nov;226:15-22. doi: 10.1016/j.vetmic.2018.10.008. Epub 2018 Oct 11. Vet Microbiol. 2018. PMID: 30389039
-
Anaplasma species of veterinary importance in Japan.Vet World. 2016 Nov;9(11):1190-1196. doi: 10.14202/vetworld.2016.1190-1196. Epub 2016 Nov 4. Vet World. 2016. PMID: 27956767 Free PMC article. Review.
-
Anaplasma marginale (Rickettsiales: Anaplasmataceae): recent advances in defining host-pathogen adaptations of a tick-borne rickettsia.Parasitology. 2004;129 Suppl:S285-300. doi: 10.1017/s0031182003004700. Parasitology. 2004. PMID: 15938516 Review.
Cited by
-
Ticks and Tick-Borne Pathogens Abound in the Cattle Population of the Rabat-Sale Kenitra Region, Morocco.Pathogens. 2021 Dec 9;10(12):1594. doi: 10.3390/pathogens10121594. Pathogens. 2021. PMID: 34959550 Free PMC article.
-
Anaplasma capra: a new emerging tick-borne zoonotic pathogen.Vet Res Commun. 2024 Jun;48(3):1329-1340. doi: 10.1007/s11259-024-10337-9. Epub 2024 Mar 1. Vet Res Commun. 2024. PMID: 38424380 Free PMC article. Review.
-
Molecular Detection of Anaplasma, Ehrlichia and Rickettsia Pathogens in Ticks Collected from Humans in the Republic of Korea, 2021.Pathogens. 2023 Jun 4;12(6):802. doi: 10.3390/pathogens12060802. Pathogens. 2023. PMID: 37375492 Free PMC article.
-
Molecular Detection of Tick-Borne Pathogens in Kumasi: With a First Report of Zoonotic Pathogens in Abattoir Workers.Biomed Res Int. 2024 Jul 14;2024:4848451. doi: 10.1155/2024/4848451. eCollection 2024. Biomed Res Int. 2024. PMID: 39035771 Free PMC article.
-
Presence of Anaplasma spp. and Their Associated Antibodies in the Swedish Goat Population.Animals (Basel). 2023 Jan 17;13(3):333. doi: 10.3390/ani13030333. Animals (Basel). 2023. PMID: 36766222 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

