Molecular Tailoring Approach for the Estimation of Intramolecular Hydrogen Bond Energy
- PMID: 34069140
- PMCID: PMC8155843
- DOI: 10.3390/molecules26102928
Molecular Tailoring Approach for the Estimation of Intramolecular Hydrogen Bond Energy
Abstract
Hydrogen bonds (HBs) play a crucial role in many physicochemical and biological processes. Theoretical methods can reliably estimate the intermolecular HB energies. However, the methods for the quantification of intramolecular HB (IHB) energy available in the literature are mostly empirical or indirect and limited only to evaluating the energy of a single HB. During the past decade, the authors have developed a direct procedure for the IHB energy estimation based on the molecular tailoring approach (MTA), a fragmentation method. This MTA-based method can yield a reliable estimate of individual IHB energy in a system containing multiple H-bonds. After explaining and illustrating the methodology of MTA, we present its use for the IHB energy estimation in molecules and clusters. We also discuss the use of this method by other researchers as a standard, state-of-the-art method for estimating IHB energy as well as those of other noncovalent interactions.
Keywords: bond energy estimation; fragmentation methods; hydrogen bond (HB); intramolecular hydrogen bond (IHB); molecular tailoring approach (MTA); noncovalent interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





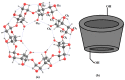


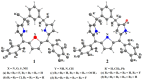
References
-
- Pauling L. The Nature of the Chemical Bond. Oxford University Press; London, UK: 1939.
-
- Jeffery G.A. An Introduction to Hydrogen Bonding. Oxford University Press; New York, NY, USA: 1997.
-
- Desiraju G.R., Steiner T. Structural Chemistry and Biology. Oxford University Press; New York, NY, USA: 1999. The Weak Hydrogen Bond.
-
- Scheiner S. Hydrogen Bond: A Theoretical Perspective. Oxford University Press; New York, NY, USA: 1997.
-
- Huggins M.L. Hydrogen Bridges in Ice and Liquid Water. J. Phys. Chem. 1936;40:723–731. doi: 10.1021/j150375a004. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

