Overexpression of Transcripts Coding for Renin-b but Not for Renin-a Reduce Oxidative Stress and Increase Cardiomyoblast Survival under Starvation Conditions
- PMID: 34069146
- PMCID: PMC8156538
- DOI: 10.3390/cells10051204
Overexpression of Transcripts Coding for Renin-b but Not for Renin-a Reduce Oxidative Stress and Increase Cardiomyoblast Survival under Starvation Conditions
Abstract
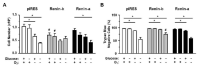
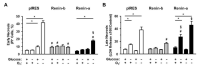
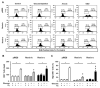
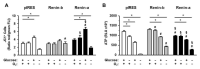
A stimulated renin-angiotensin system is known to promote oxidative stress, apoptosis, necrosis and fibrosis. Renin transcripts (renin-b; renin-c) encoding a cytosolic renin isoform have been discovered that may in contrast to the commonly known secretory renin (renin-a) exert protective effects Here, we analyzed the effect of renin-a and renin-b overexpression in H9c2 cardiomyoblasts on apoptosis and necrosis as well as on potential mechanisms involved in cell death processes. To mimic ischemic conditions, cells were exposed to glucose starvation, anoxia or combined oxygen-glucose deprivation (OGD) for 24 h. Under OGD, control cells exhibited markedly increased necrotic and apoptotic cell death accompanied by enhanced ROS accumulation, loss of mitochondrial membrane potential and decreased ATP levels. The effects of OGD on necrosis were exaggerated in renin-a cells, but markedly diminished in renin-b cells. However, with respect to apoptosis, the effects of OGD were almost completely abolished in renin-b cells but interestingly also moderately diminished in renin-a cells. Under glucose depletion we found opposing responses between renin-a and renin-b cells; while the rate of necrosis and apoptosis was aggravated in renin-a cells, it was attenuated in renin-b cells. Based on our results, strategies targeting the regulation of cytosolic renin-b as well as the identification of pathways involved in the protective effects of renin-b may be helpful to improve the treatment of ischemia-relevant diseases.
Keywords: ATP levels; cardiac H9c2 cells; cytosolic renin; ischemia-induced cell death; mitochondrial membrane potential; reactive oxygen species; secretory renin.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




Similar articles
-
Overexpression of Renin-B Induces Warburg-like Effects That Are Associated with Increased AKT/mTOR Signaling.Cells. 2022 Apr 26;11(9):1459. doi: 10.3390/cells11091459. Cells. 2022. PMID: 35563765 Free PMC article.
-
Non-secretory renin reduces oxidative stress and increases cardiomyoblast survival during glucose and oxygen deprivation.Sci Rep. 2020 Feb 11;10(1):2329. doi: 10.1038/s41598-020-59216-8. Sci Rep. 2020. PMID: 32047214 Free PMC article.
-
Anti-necrotic and cardioprotective effects of a cytosolic renin isoform under ischemia-related conditions.J Mol Med (Berl). 2016 Jan;94(1):61-9. doi: 10.1007/s00109-015-1321-z. Epub 2015 Aug 11. J Mol Med (Berl). 2016. PMID: 26256830
-
Cytosolic renin is targeted to mitochondria and induces apoptosis in H9c2 rat cardiomyoblasts.J Cell Mol Med. 2009 Sep;13(9A):2926-37. doi: 10.1111/j.1582-4934.2008.00448.x. Epub 2008 Jul 30. J Cell Mol Med. 2009. PMID: 18671756 Free PMC article.
-
An Alternative Promoter in Intron1 of the Renin Gene is Regulated by Glucose Starvation via Serum Response Factor.Cell Physiol Biochem. 2017;42(4):1447-1457. doi: 10.1159/000479209. Epub 2017 Jul 17. Cell Physiol Biochem. 2017. PMID: 28715805
Cited by
-
Case-inspired exploration of renin mutations in autosomal dominant tubulointerstitial kidney disease: not all paths lead to the endoplasmic reticulum.Pediatr Nephrol. 2024 Aug;39(8):2363-2375. doi: 10.1007/s00467-024-06350-4. Epub 2024 Mar 23. Pediatr Nephrol. 2024. PMID: 38520530
-
Overexpression of Renin-B Induces Warburg-like Effects That Are Associated with Increased AKT/mTOR Signaling.Cells. 2022 Apr 26;11(9):1459. doi: 10.3390/cells11091459. Cells. 2022. PMID: 35563765 Free PMC article.
-
Renin-a in the Subfornical Organ Plays a Critical Role in the Maintenance of Salt-Sensitive Hypertension.Biomolecules. 2022 Aug 24;12(9):1169. doi: 10.3390/biom12091169. Biomolecules. 2022. PMID: 36139008 Free PMC article.
-
PGRMC1 Ablation Protects from Energy-Starved Heart Failure by Promoting Fatty Acid/Pyruvate Oxidation.Cells. 2023 Feb 27;12(5):752. doi: 10.3390/cells12050752. Cells. 2023. PMID: 36899888 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

