MicroRNAs as Epigenetic Determinants of Treatment Response and Potential Therapeutic Targets in Prostate Cancer
- PMID: 34069147
- PMCID: PMC8156532
- DOI: 10.3390/cancers13102380
MicroRNAs as Epigenetic Determinants of Treatment Response and Potential Therapeutic Targets in Prostate Cancer
Abstract
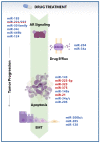
Prostate cancer (PCa) is the second most common tumor in men worldwide, and the fifth leading cause of male cancer-related deaths in western countries. PC is a very heterogeneous disease, meaning that optimal clinical management of individual patients is challenging. Depending on disease grade and stage, patients can be followed in active surveillance protocols or undergo surgery, radiotherapy, hormonal therapy, and chemotherapy. Although therapeutic advancements exist in both radiatiotherapy and chemotherapy, in a considerable proportion of patients, the treatment remains unsuccessful, mainly due to tumor poor responsiveness and/or recurrence and metastasis. microRNAs (miRNAs), small noncoding RNAs that epigenetically regulate gene expression, are essential actors in multiple tumor-related processes, including apoptosis, cell growth and proliferation, autophagy, epithelial-to-mesenchymal transition, invasion, and metastasis. Given that these processes are deeply involved in cell response to anti-cancer treatments, miRNAs have been considered as key determinants of tumor treatment response. In this review, we provide an overview on main PCa-related miRNAs and describe the biological mechanisms by which specific miRNAs concur to determine PCa response to radiation and drug therapy. Additionally, we illustrate whether miRNAs can be considered novel therapeutic targets or tools on the basis of the consequences of their expression modulation in PCa experimental models.
Keywords: epigenetics; microRNA; prostate cancer; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mottet N., van den Bergh R.C.N., Briers E., van den Broeck T., Cumberbatch M.G., de Santis M., Fanti S., Fossati N., Gandaglia G., Gillessen S., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. - DOI - PubMed
-
- Bjurlin M.A., Carter H.B., Schellhammer P., Cookson M.S., Gomella L.G., Troyer D., Wheeler T.M., Schlossberg S., Penson D.F., Taneja S.S. Optimization of initial prostate biopsy in clinical practice: Sampling, labeling and specimen processing. J. Urol. 2013;189:2039–2046. doi: 10.1016/j.juro.2013.02.072. - DOI - PMC - PubMed
-
- Cornford P., van den Bergh R.C.N., Briers E., van den Broeck T., Cumberbatch M.G., de Santis M., Fanti S., Fossati N., Gandaglia G., Gillessen S., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

