Acute Myeloid Leukemia: Is It T Time?
- PMID: 34069204
- PMCID: PMC8156992
- DOI: 10.3390/cancers13102385
Acute Myeloid Leukemia: Is It T Time?
Abstract
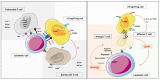
Acute myeloid leukemia (AML) is a heterogeneous disease driven by impaired differentiation of hematopoietic primitive cells toward myeloid lineages (monocytes, granulocytes, red blood cells, platelets), leading to expansion and accumulation of "stem" and/or "progenitor"-like or differentiated leukemic cells in the bone marrow and blood. AML progression alters the bone marrow microenvironment and inhibits hematopoiesis' proper functioning, causing sustained cytopenia and immunodeficiency. This review describes how the AML microenvironment influences lymphoid lineages, particularly T lymphocytes that originate from the thymus and orchestrate adaptive immune response. We focus on the elderly population, which is mainly affected by this pathology. We discuss how a permissive AML microenvironment can alter and even worsen the thymic function, T cells' peripheral homeostasis, phenotype, and functions. Based on the recent findings on the mechanisms supporting that AML induces quantitative and qualitative changes in T cells, we suggest and summarize current immunotherapeutic strategies and challenges to overcome these anomalies to improve the anti-leukemic immune response and the clinical outcome of patients.
Keywords: T cells; acute myeloid leukemia; immunotherapy; thymus function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The potential role of the thymus in immunotherapies for acute myeloid leukemia.Front Immunol. 2023 Feb 6;14:1102517. doi: 10.3389/fimmu.2023.1102517. eCollection 2023. Front Immunol. 2023. PMID: 36814919 Free PMC article. Review.
-
Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia.Hematology. 2018 Dec;23(10):729-739. doi: 10.1080/10245332.2018.1486064. Epub 2018 Jun 14. Hematology. 2018. PMID: 29902132 Review.
-
Adipogenic Mesenchymal Stromal Cells from Bone Marrow and Their Hematopoietic Supportive Role: Towards Understanding the Permissive Marrow Microenvironment in Acute Myeloid Leukemia.Stem Cell Rev Rep. 2016 Apr;12(2):235-44. doi: 10.1007/s12015-015-9639-z. Stem Cell Rev Rep. 2016. PMID: 26649729
-
Physician Education: Myelodysplastic Syndrome.Oncologist. 1996;1(4):284-287. Oncologist. 1996. PMID: 10388004
-
Exploiting epigenetically mediated changes: Acute myeloid leukemia, leukemia stem cells and the bone marrow microenvironment.Adv Cancer Res. 2019;141:213-253. doi: 10.1016/bs.acr.2018.12.005. Epub 2019 Jan 21. Adv Cancer Res. 2019. PMID: 30691684 Review.
Cited by
-
LINC00998 functions as a novel tumor suppressor in acute myeloid leukemia via regulating the ZFP36 ring finger protein/mammalian target of rapamycin complex 2 axis.Bioengineered. 2021 Dec;12(2):10363-10372. doi: 10.1080/21655979.2021.1996506. Bioengineered. 2021. PMID: 34699314 Free PMC article.
-
The potential role of the thymus in immunotherapies for acute myeloid leukemia.Front Immunol. 2023 Feb 6;14:1102517. doi: 10.3389/fimmu.2023.1102517. eCollection 2023. Front Immunol. 2023. PMID: 36814919 Free PMC article. Review.
-
Enhanced MCM5 Level Predicts Bad Prognosis in Acute Myeloid Leukemia.Mol Biotechnol. 2023 Aug;65(8):1242-1252. doi: 10.1007/s12033-022-00623-9. Epub 2022 Dec 7. Mol Biotechnol. 2023. PMID: 36479666 Free PMC article.
-
Blood metabolic and physiological profiles of Bama miniature pigs at different growth stages.Porcine Health Manag. 2022 Aug 8;8(1):35. doi: 10.1186/s40813-022-00278-7. Porcine Health Manag. 2022. PMID: 35941611 Free PMC article.
-
Chronic inflammation deters natural killer cell fitness and cytotoxicity in myeloid leukemia.Blood Adv. 2025 Feb 25;9(4):759-773. doi: 10.1182/bloodadvances.2024014592. Blood Adv. 2025. PMID: 39571169 Free PMC article.
References
-
- Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

