Horse Placental Extract Enhances Neurogenesis in the Presence of Amyloid β
- PMID: 34069207
- PMCID: PMC8157028
- DOI: 10.3390/nu13051672
Horse Placental Extract Enhances Neurogenesis in the Presence of Amyloid β
Abstract
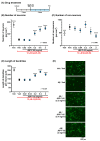
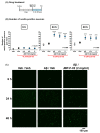
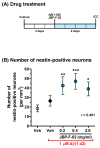
Human placental extract and animal-derived placental extracts from pigs and horses host a wide range of biological activities. Several placental products are used as medicines, cosmetics, and healthcare substances worldwide. However, the use of placental extracts for neuronal functioning is currently not established because the number of relevant studies is limited. A few previous reports suggested the neuroprotective effect and dendrite genesis effect of placental extract. However, no studies have reported on neurogenesis in placental extracts. Therefore, we aimed to investigate the effects of horse placental extract on neurogenesis, and we examined the protective effect of the extract on the onset of memory disorder. A horse placental extract, JBP-F-02, was used in this study. JBP-F-02 treatment dose-dependently increased the number of neural stem cells and dendrite length under Aβ treatment in primary cultured cortical cells. The oral administration of JBP-F-02 to a 5XFAD mouse model of Alzheimer's disease at a young age significantly prevented the onset of memory dysfunction. This study suggests that the extract has the potential to prevent dementia.
Keywords: Alzheimer’s disease; dendrite; horse placental extract; memory recovery; neurogenesis.
Conflict of interest statement
A.T. and E.H. are employees of Japan Bio Products.
Figures

Similar articles
-
Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice.J Alzheimers Dis. 2014;41(1):233-41. doi: 10.3233/JAD-132417. J Alzheimers Dis. 2014. PMID: 24583405
-
Shenzao jiannao oral liquid, an herbal formula, ameliorates cognitive impairments by rescuing neuronal death and triggering endogenous neurogenesis in AD-like mice induced by a combination of Aβ42 and scopolamine.J Ethnopharmacol. 2020 Sep 15;259:112957. doi: 10.1016/j.jep.2020.112957. Epub 2020 May 19. J Ethnopharmacol. 2020. PMID: 32416248
-
Crocus sativus Extract Tightens the Blood-Brain Barrier, Reduces Amyloid β Load and Related Toxicity in 5XFAD Mice.ACS Chem Neurosci. 2017 Aug 16;8(8):1756-1766. doi: 10.1021/acschemneuro.7b00101. Epub 2017 May 15. ACS Chem Neurosci. 2017. PMID: 28471166 Free PMC article.
-
Icariin decreases both APP and Aβ levels and increases neurogenesis in the brain of Tg2576 mice.Neuroscience. 2015 Sep 24;304:29-35. doi: 10.1016/j.neuroscience.2015.06.010. Epub 2015 Jun 12. Neuroscience. 2015. PMID: 26079110
-
Leaf and stem of Vitis amurensis and its active components protect against amyloid β protein (25-35)-induced neurotoxicity.Arch Pharm Res. 2010 Oct;33(10):1655-64. doi: 10.1007/s12272-010-1015-6. Epub 2010 Oct 30. Arch Pharm Res. 2010. PMID: 21052941
Cited by
-
Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report.Open Vet J. 2023 May;13(5):668-676. doi: 10.5455/OVJ.2023.v13.i5.21. Epub 2023 May 27. Open Vet J. 2023. PMID: 37304605 Free PMC article.
-
Porcine Placental Extract Improves the Lipid Profile and Body Weight in a Post-Menopausal Rat Model Without Affecting Reproductive Tissues.Nutrients. 2025 Mar 11;17(6):984. doi: 10.3390/nu17060984. Nutrients. 2025. PMID: 40290014 Free PMC article.
-
Equine placental extract supplement as a night barking remedy in dogs with cognitive dysfunction syndrome.Vet Med Sci. 2022 Sep;8(5):1887-1892. doi: 10.1002/vms3.893. Epub 2022 Aug 3. Vet Med Sci. 2022. PMID: 35921448 Free PMC article.
References
-
- Takuma K., Mizoguchi H., Funatsu Y., Kitahara Y., Ibi D., Kamei H., Matsuda T., Koike K., Inoue M., Nagai T., et al. Placental Extract Improves Hippocampal Neuronal Loss and Fear Memory Impairment Resulting from Chronic Restraint Stress in Ovariectomized Mice. J. Pharmacol. Sci. 2012;120:89–97. doi: 10.1254/jphs.12115FP. - DOI - PMC - PubMed
-
- Kogure C., Tohda C. Human Placenta Extract Ameliorates Memory Dysfunction and Dendritic Atrophy in a 5XFAD Mouse Model of A Lzheimer’s Disease. Tradit. Kamp. Med. 2017;4:94–104. doi: 10.1002/tkm2.1075. - DOI
-
- Oakley H., Cole S.L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., et al. Intraneuronal Beta-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

