1,2,3-Triazoles as Biomimetics in Peptide Science
- PMID: 34069302
- PMCID: PMC8156386
- DOI: 10.3390/molecules26102937
1,2,3-Triazoles as Biomimetics in Peptide Science
Abstract
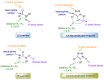
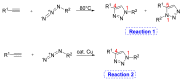
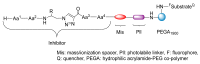
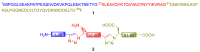
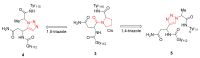
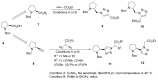
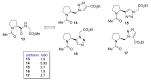
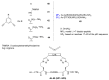
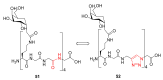
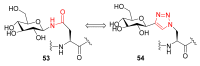
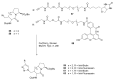
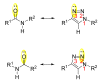
Natural peptides are an important class of chemical mediators, essential for most vital processes. What limits the potential of the use of peptides as drugs is their low bioavailability and enzymatic degradation in vivo. To overcome this limitation, the development of new molecules mimicking peptides is of great importance for the development of new biologically active molecules. Therefore, replacing the amide bond in a peptide with a heterocyclic bioisostere, such as the 1,2,3-triazole ring, can be considered an effective solution for the synthesis of biologically relevant peptidomimetics. These 1,2,3-triazoles may have an interesting biological activity, because they behave as rigid link units, which can mimic the electronic properties of amide bonds and show bioisosteric effects. Additionally, triazole can be used as a linker moiety to link peptides to other functional groups.
Keywords: 1,2,3-triazole; CuAAC; amide bond; bioisostere; click chemistry; peptidomimetic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
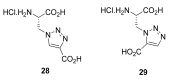
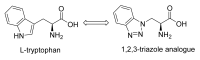
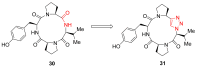


Similar articles
-
1,4-Disubstituted 1,2,3-Triazoles as Amide Bond Surrogates for the Stabilisation of Linear Peptides with Biological Activity.Molecules. 2020 Aug 6;25(16):3576. doi: 10.3390/molecules25163576. Molecules. 2020. PMID: 32781656 Free PMC article. Review.
-
1,5-Disubstituted 1,2,3-triazoles: Molecular scaffolds for medicinal chemistry and biomolecular mimetics.Eur J Med Chem. 2025 Jul 5;291:117614. doi: 10.1016/j.ejmech.2025.117614. Epub 2025 Apr 10. Eur J Med Chem. 2025. PMID: 40239486 Review.
-
1,2,3-Triazoles as amide-bond surrogates in peptidomimetics.Chimia (Aarau). 2013;67(4):262-6. doi: 10.2533/chimia.2013.262. Chimia (Aarau). 2013. PMID: 23967702 Review.
-
Click Chemistry for Cyclic Peptide Drug Design.Methods Mol Biol. 2019;2001:133-145. doi: 10.1007/978-1-4939-9504-2_8. Methods Mol Biol. 2019. PMID: 31134571
-
Facets of click-mediated triazoles in decorating amino acids and peptides.Chem Commun (Camb). 2025 Jan 7;61(4):639-657. doi: 10.1039/d4cc03887h. Chem Commun (Camb). 2025. PMID: 39552572 Review.
Cited by
-
Rational Design and Synthesis of New Selective COX-2 Inhibitors with In Vivo PGE2-Lowering Activity by Tethering Benzenesulfonamide and 1,2,3-Triazole Pharmacophores to Some NSAIDs.Pharmaceuticals (Basel). 2022 Sep 20;15(10):1165. doi: 10.3390/ph15101165. Pharmaceuticals (Basel). 2022. PMID: 36297278 Free PMC article.
-
MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction.Nanomaterials (Basel). 2022 Mar 24;12(7):1070. doi: 10.3390/nano12071070. Nanomaterials (Basel). 2022. PMID: 35407188 Free PMC article.
-
In Vitro Antioxidant and Pancreatic Anticancer Activity of Novel 5-Fluorouracil-Coumarin Conjugates.Pharmaceutics. 2022 Oct 10;14(10):2152. doi: 10.3390/pharmaceutics14102152. Pharmaceutics. 2022. PMID: 36297585 Free PMC article.
-
Red light-emitting short Mango-based system enables tracking a mycobacterial small noncoding RNA in infected macrophages.Nucleic Acids Res. 2023 Apr 11;51(6):2586-2601. doi: 10.1093/nar/gkad100. Nucleic Acids Res. 2023. PMID: 36840712 Free PMC article.
-
1,2,3-Triazole Tethered Hybrid Capsaicinoids as Antiproliferative Agents Active against Lung Cancer Cells (A549).ACS Omega. 2022 Sep 1;7(36):32078-32100. doi: 10.1021/acsomega.2c03325. eCollection 2022 Sep 13. ACS Omega. 2022. PMID: 36119972 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

