Recombinant γY278H Fibrinogen Showed Normal Secretion from CHO Cells, but a Corresponding Heterozygous Patient Showed Hypofibrinogenemia
- PMID: 34069309
- PMCID: PMC8156302
- DOI: 10.3390/ijms22105218
Recombinant γY278H Fibrinogen Showed Normal Secretion from CHO Cells, but a Corresponding Heterozygous Patient Showed Hypofibrinogenemia
Abstract
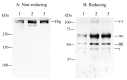
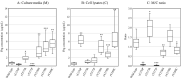
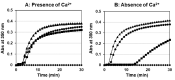
We identified a novel heterozygous hypofibrinogenemia, γY278H (Hiroshima). To demonstrate the cause of reduced plasma fibrinogen levels (functional level: 1.12 g/L and antigenic level: 1.16 g/L), we established γY278H fibrinogen-producing Chinese hamster ovary (CHO) cells. An enzyme-linked immunosorbent assay demonstrated that synthesis of γY278H fibrinogen inside CHO cells and secretion into the culture media were not reduced. Then, we established an additional five variant fibrinogen-producing CHO cell lines (γL276P, γT277P, γT277R, γA279D, and γY280C) and conducted further investigations. We have already established 33 γ-module variant fibrinogen-producing CHO cell lines, including 6 cell lines in this study, but only the γY278H and γT277R cell lines showed disagreement, namely, recombinant fibrinogen production was not reduced but the patients' plasma fibrinogen level was reduced. Finally, we performed fibrinogen degradation assays and demonstrated that the γY278H and γT277R fibrinogens were easily cleaved by plasmin whereas their polymerization in the presence of Ca2+ and "D:D" interaction was normal. In conclusion, our investigation suggested that patient γY278H showed hypofibrinogenemia because γY278H fibrinogen was secreted normally from the patient's hepatocytes but then underwent accelerated degradation by plasmin in the circulation.
Keywords: Chinese hamster ovary cells; congenital fibrinogen disorders; hypofibrinogenemia; plasmin degradation; recombinant fibrinogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
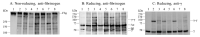



Similar articles
-
Recombinant γT305A fibrinogen indicates severely impaired fibrin polymerization due to the aberrant function of hole 'A' and calcium binding sites.Thromb Res. 2014 Aug;134(2):518-25. doi: 10.1016/j.thromres.2014.06.002. Epub 2014 Jun 11. Thromb Res. 2014. PMID: 24968960
-
Heterozygous variant fibrinogen γA289V (Kanazawa III) was confirmed as hypodysfibrinogenemia by plasma and recombinant fibrinogens.Int J Lab Hematol. 2020 Apr;42(2):190-197. doi: 10.1111/ijlh.13152. Epub 2020 Jan 20. Int J Lab Hematol. 2020. PMID: 31957968
-
Differences in the function and secretion of congenital aberrant fibrinogenemia between heterozygous γD320G (Okayama II) and γΔN319-ΔD320 (Otsu I).Thromb Res. 2015 Dec;136(6):1318-24. doi: 10.1016/j.thromres.2015.11.011. Epub 2015 Nov 10. Thromb Res. 2015. PMID: 26573395
-
Diagnosis of congenital fibrinogen disorders.Ann Biol Clin (Paris). 2016 Aug 1;74(4):405-12. doi: 10.1684/abc.2016.1167. Ann Biol Clin (Paris). 2016. PMID: 27492693 Review. English.
-
From Routine to Research Laboratory: Strategies for the Diagnosis of Congenital Fibrinogen Disorders.Hamostaseologie. 2020 Nov;40(4):460-466. doi: 10.1055/a-1182-3510. Epub 2020 Jul 9. Hamostaseologie. 2020. PMID: 32645726 Review.
References
-
- Medved L., Weisel J.W. Fibrinogen and Factor XIII Subcommittee of Scientific Standardization Committee of International Society on Thrombosis and Haemostasis. Recommendations for nomenclature on fibrinogen and fibrin. J. Thromb. Haemost. 2009;7:355–359. doi: 10.1111/j.1538-7836.2008.03242.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

