Synergistic Interaction between 5-FU and an Analog of Sulforaphane-2-Oxohexyl Isothiocyanate-In an In Vitro Colon Cancer Model
- PMID: 34069385
- PMCID: PMC8158758
- DOI: 10.3390/molecules26103019
Synergistic Interaction between 5-FU and an Analog of Sulforaphane-2-Oxohexyl Isothiocyanate-In an In Vitro Colon Cancer Model
Abstract
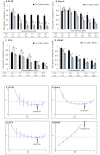
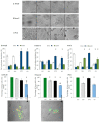
Combination therapy is based on the beneficial effects of pharmacodynamic interaction (synergistic or additive) between combined drugs or substances. A considerable group of candidates for combined treatments are natural compounds (e.g., isothiocyanates) and their analogs, which are tested in combination with anticancer drugs. We tested the anticancer effect of the combined treatment of isothiocyanate 2-oxohexyl isothiocyanate and 5-fluorouracil in colon and prostate cancer cell lines. The type of interaction was described using the Chou-Talalay method. The cytostatic and cytotoxic activities of the most promising combined treatments were investigated. In conclusion, we showed that combined treatment with 5-fluorouracil and 2-oxohexyl isothiocyanate acted synergistically in colon cancer. This activity is dependent on the cytostatic properties of the tested compounds and leads to the intensification of their individual cytotoxic activity. The apoptotic process is considered to be the main mechanism of cytotoxicity in this combined treatment.
Keywords: 5-fluorouracil; isothiocyanates; sulforaphane; synergistic effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
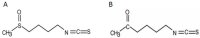


Similar articles
-
In Vitro Evaluation of Sulforaphane and a Natural Analog as Potent Inducers of 5-Fluorouracil Anticancer Activity.Molecules. 2018 Nov 21;23(11):3040. doi: 10.3390/molecules23113040. Molecules. 2018. PMID: 30469330 Free PMC article.
-
Combination treatment with 5-fluorouracil and isothiocyanates shows an antagonistic effect in Chinese hamster fibroblast cells line-V79.Acta Pol Pharm. 2011 May-Jun;68(3):331-42. Acta Pol Pharm. 2011. PMID: 21648187
-
Autophagic cell death and premature senescence: New mechanism of 5-fluorouracil and sulforaphane synergistic anticancer effect in MDA-MB-231 triple negative breast cancer cell line.Food Chem Toxicol. 2018 Jan;111:1-8. doi: 10.1016/j.fct.2017.10.056. Epub 2017 Nov 2. Food Chem Toxicol. 2018. PMID: 29104175
-
Sulforaphane as an anticancer molecule: mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems.Arch Pharm Res. 2020 Apr;43(4):371-384. doi: 10.1007/s12272-020-01225-2. Epub 2020 Mar 10. Arch Pharm Res. 2020. PMID: 32152852 Review.
-
Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity.Molecules. 2022 Mar 7;27(5):1750. doi: 10.3390/molecules27051750. Molecules. 2022. PMID: 35268851 Free PMC article. Review.
Cited by
-
Diosmetin Exerts Synergistic Effects in Combination with 5-Fluorouracil in Colorectal Cancer Cells.Biomedicines. 2022 Feb 24;10(3):531. doi: 10.3390/biomedicines10030531. Biomedicines. 2022. PMID: 35327333 Free PMC article.
-
Unveiling a sustainable approach to cancer treatment: the antitumor activity of amygdalin and cell-free supernatant of Lacticaseibacillus rhamnosus.Folia Microbiol (Praha). 2025 Jul 10. doi: 10.1007/s12223-025-01289-x. Online ahead of print. Folia Microbiol (Praha). 2025. PMID: 40640516
-
In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells.Curr Oncol. 2023 Jun 27;30(7):6197-6219. doi: 10.3390/curroncol30070460. Curr Oncol. 2023. PMID: 37504320 Free PMC article.
-
Organosulfur Compounds in Colorectal Cancer Prevention and Progression.Nutrients. 2024 Mar 11;16(6):802. doi: 10.3390/nu16060802. Nutrients. 2024. PMID: 38542713 Free PMC article. Review.
-
Graphene Oxide Enhanced Cisplatin Cytotoxic Effect in Glioblastoma and Cervical Cancer.Molecules. 2023 Aug 25;28(17):6253. doi: 10.3390/molecules28176253. Molecules. 2023. PMID: 37687081 Free PMC article.
References
-
- Yang Y., Ma L., Xu Y., Liu Y., Li W., Cai J., Zhang Y. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020;11 doi: 10.1038/s41419-020-2675-x. - DOI - PMC - PubMed
-
- Thirion P., Michiels S., Pignon J.P., Buyse M., Braud A.C., Carlson R.W., O’Connell M., Sargent P., Piedbois P., Meta-Analysis Group in Cancer et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: An updated meta-analysis. J. Clin. Oncol. 2004;22:3766–3775. doi: 10.1200/jco.2004.03.104. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

