17-AAG-Induced Activation of the Autophagic Pathway in Leishmania Is Associated with Parasite Death
- PMID: 34069389
- PMCID: PMC8158731
- DOI: 10.3390/microorganisms9051089
17-AAG-Induced Activation of the Autophagic Pathway in Leishmania Is Associated with Parasite Death
Abstract
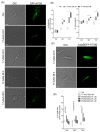
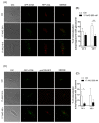
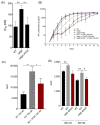
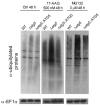
The heat shock protein 90 (Hsp90) is thought to be an excellent drug target against parasitic diseases. The leishmanicidal effect of an Hsp90 inhibitor, 17-N-allylamino-17-demethoxygeldanamycin (17-AAG), was previously demonstrated in both in vitro and in vivo models of cutaneous leishmaniasis. Parasite death was shown to occur in association with severe ultrastructural alterations in Leishmania, suggestive of autophagic activation. We hypothesized that 17-AAG treatment results in the abnormal activation of the autophagic pathway, leading to parasite death. To elucidate this process, experiments were performed using transgenic parasites with GFP-ATG8-labelled autophagosomes. Mutant parasites treated with 17-AAG exhibited autophagosomes that did not entrap cargo, such as glycosomes, or fuse with lysosomes. ATG5-knockout (Δatg5) parasites, which are incapable of forming autophagosomes, demonstrated lower sensitivity to 17-AAG-induced cell death when compared to wild-type (WT) Leishmania, further supporting the role of autophagy in 17-AAG-induced cell death. In addition, Hsp90 inhibition resulted in greater accumulation of ubiquitylated proteins in both WT- and Δatg5-treated parasites compared to controls, in the absence of proteasome overload. In conjunction with previously described ultrastructural alterations, herein we present evidence that treatment with 17-AAG causes abnormal activation of the autophagic pathway, resulting in the formation of immature autophagosomes and, consequently, incidental parasite death.
Keywords: Hsp90; Hsp90 inhibitors; autophagy; chemotherapy; leishmaniasis; ubiquitin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

